ABSTRACT
Maternal diabetes has been demonstrated to adversely affect oocyte quality in mouse oocytes. However, the potential molecular mechanisms are poorly understood. Here, we established a type I diabetic mouse model and detected the increased reactive oxygen species (ROS) levels and decreased Sirt3 expression in oocytes from diabetic mice. Furthermore, we found that forced expression of Sirt3 in diabetic oocytes significantly attenuates such an excessive production of ROS. The acetylation status of lysine 68 of superoxide dismutase (SOD2K68) is dependent on Sirt3 in oocytes. In line with this, SOD2K68 acetylation levels were markedly increased in diabetic oocytes, and Sirt3 overexpression could effectively suppress this tendency. Importantly, the deacetylation-mimetic mutant SOD2K68R is capable of partly preventing the oxidative stress in oocytes from diabetic mice. In conclusion, our findings support a model where Sirt3 plays a protective role against oxidative stress in oocytes exposed to maternal diabetes through deacetylating SOD2K68.
KEYWORDS: diabetes, oocyte, oxidative stress, Sirtuin, SOD
Introduction
Women with poorly controlled insulin-dependent (type I) diabetes often experience a higher incidence of reproductive problems, such as spontaneous abortions, neonatal morbidity and mortality, and congenital malformations.1,2 Earlier studies showed that the diabetic condition is detrimental to pre-and post-implantation embryo development in rodents.3-5 Maternal hyperglycemia adversely affects the progression from the one-cell stage to blastocyst stage and about half of 2-cell embryos isolated from sub-diabetic females were unable to develop to 8-cell stage even in non-diabetic maternal environment.5,6 Emerging evidence has shown that these effects are associated with compromised oocyte maturation. Oocytes from diabetic mice experience delayed germinal vesicle (GV) breakdown, abnormal cellular metabolism, and mitochondrial dysfunction.3,7-9 Mitochondria are important and pervasive source of reactive oxygen species (ROS), as a by-product of oxidative phosphorylation in the cell.10 Mitochondrial dysfunction inevitably enhances ROS production, resulting in oxidative stress. Oocyte maturation depends on the homeostasis between the production of ROS and antioxidants. Over-production of ROS induces oxidative damage to the cells, including membrane lipids, proteins, as well as DNA.11,12 Notably, maternal hyperglycemia is known to increase oxidative stress in diverse tissues.13 Moreover, such oxidative damage has been shown to influence the ability of oocyte maturation, fertilization, and pregnancy rates in mice as well as in humans.14
Sirtuins (Sirts) are a highly conserved family of proteins possessing NAD+-dependent protein deacetylase activity. Mammals have 7 members (Sirt1-Sirt7) displayed differential cellular localization and function in numerous physiologic processes.15 Among them, Sirt3 is localized in mitochondria and controls the acetylation status of almost 80∼90% of mitochondrial proteins.16 It regulates the production of ROS via the electron transport chain, as well as the detoxification of ROS through activation of antioxidant enzymes.15,17,18 Recent studies highlight the critical role of Sirt3 in modulating ROS homeostasis in response to caloric restriction. Sirt3 is able to directly deacetylates isocitrate dehydrogenase 2 (IDH2), increasing the levels of NADPH and thereupon protecting cells from oxidative stress.17 In addition, we and others found that Sirt3 also participates in controlling ROS homeostasis during oocyte maturation and fertilization in vitro.19,20 Superoxide dismutases (SODs) are a class of enzymes that catalyze the detoxification of superoxide into oxygen and hydrogen peroxide, and then converted to oxygen and water by catalase. Mammalian cells express 3 forms of SOD (SOD1–3). Of these 3 forms, mitochondria SOD2 is an antioxidant enzyme and plays a crucial role in controlling ROS production.21 Previous studies have demonstrated that SOD2 activity is strongly regulated by the acetylation at several conserved lysine residues.18 Acetylation levels of SOD2 are negatively associated with its enzymatic activity. Elevated ROS production stimulates Sirt3 to deacetylate SOD2, consequently activating SOD2 and clearing ROS.18,21,22 Sirt3 has also been shown to interact with FOXO3a to regulate SOD2 and catalase, positively affecting disorders.23
Numerous studies have reported that oocytes from diabetes mice have metabolic dysfunction and meiotic defects,24 but the underlying mechanisms remain to be discovered. In this study, we used a STZ-induced diabetic mouse model to investigate whether Sirt3 is a factor contributing to the poor quality of oocytes from diabetic mice.
Results
Increased ROS levels in oocytes from diabetic mice
We have shown that maternal diabetes results in spatial, structural and metabolic alterations in oocyte mitochondria.25 Given that mitochondria are the major source of ROS in the cell,17 we therefore decided to evaluate the generation of ROS in oocytes from control and diabetic mice. Both GV and ovulated oocytes (MII) were collected and stained with 5-(and-6)-chloromethyl-2ˊ,7ˊ-dichlorodihydrofluorescein diacetate (CM-H2DCFAD). Our quantitative analysis revealed that the relative fluorescence intensity in both stage oocytes from diabetic mice was significantly higher than their counterparts from control mice (Fig. 1). These results indicate that ROS generation was increased when oocytes exposed to hyperglycemic environment.
Figure 1.
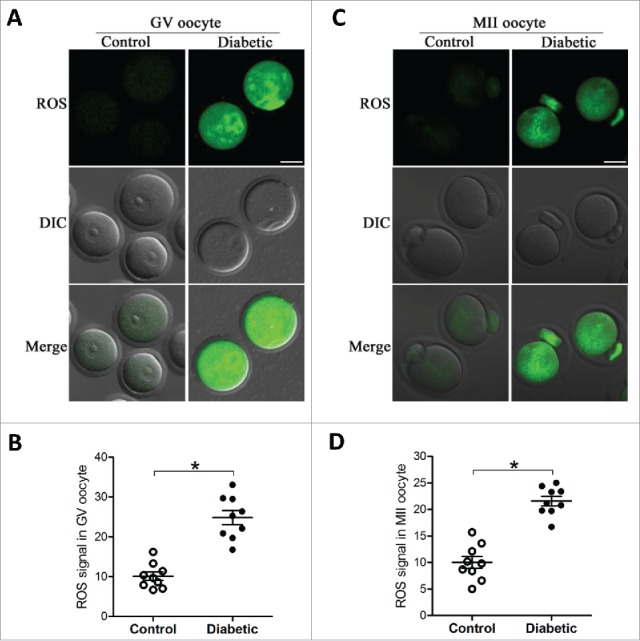
Increased ROS levels in oocyte from diabetes mice. GV and MII oocytes collected from control and diabetic mice were stained by CM-H2DCFAD (green) to evaluate ROS levels. (A and C) Representative images of CM-H2DCFAD fluorescence in oocytes from control and diabetic mice. Scale bar: 25 μm. (B and D) Quantitative analysis of fluorescence intensity shown in A and C (n = 9 oocytes for each group). Data were expressed as mean ± SD from 3 independent experiments. *p < 0.05 vs control.
Reduction of Sirt3 expression in oocytes from diabetic mice
Sirt3 is involved in regulating the global acetylation of mitochondrial proteins,26 and identified as a pivotal modulator of oxidative stress by deacetylation of substrates.18 Based on these findings, we explored whether Sirt3 expression was altered in oocytes from diabetic mice. By performing Western blotting, we found that the content of Sirt3 protein was dramatically decreased as compared with controls (Fig. 2), implying that the elevated ROS levels in diabetic oocytes may be associated with the reduction of Sirt3 expression.
Figure 2.
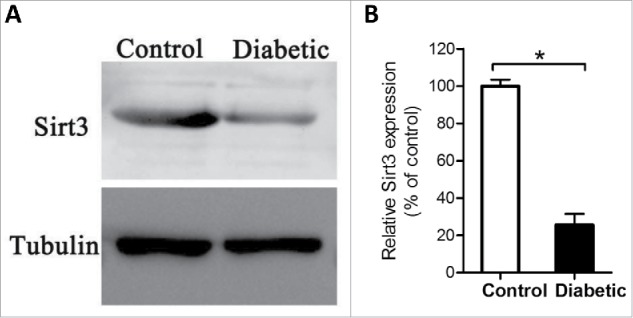
Reduced Sirt3 expression in oocyte from diabetes mice. Fully-grown GV oocytes were collected from control and diabetic mice, and then processed for immunoblotting. (A) Western blot analysis showed the reduced Sirt3 expression in oocytes from diabetic mice compared with controls. Tubulin served as an internal control. (B) Band intensity was measured by Image J software, and the ratio of Sirt3/Tubulin expression was normalized. All protein gel blot experiments were repeated at least 3 times, with a representative gel image shown.
Sirt3 overexpression attenuates ROS production in oocytes from diabetic mice
We previously demonstrated that knockdown of endogenous Sirt3 protein in normal oocytes could elevate the ROS levels in oocytes apparently.19 Here, to test whether the observed increased ROS levels in diabetic oocytes is due to Sirt3 reduction, we decided to elevate the Sirt3 expression and then assess the ROS content. To do this, we performed an overexpression experiment by injecting exogenous Myc-Sirt3 mRNA into fully-grown oocytes from diabetic mice, PBS was injected as negative control. Following in vitro-maturation, MII oocytes were collected for further analysis. After staining with CM-H2DCFAD fluorescent dye, quantitative analysis revealed that forced expression of Sirt3 markedly reduced the ROS levels in diabetic MII oocytes, as shown in Fig. 3. These data provide a critical evidence that loss of Sirt3 protein is a major factor leading to oxidative stress in oocytes from diabetic mice.
Figure 3.
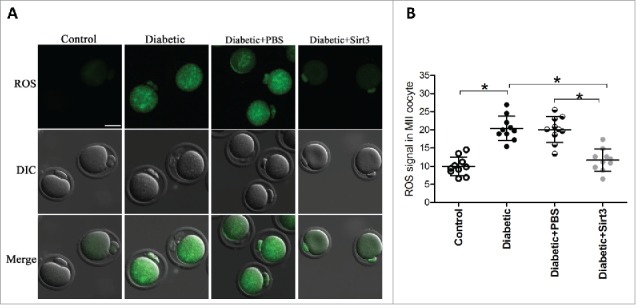
Sirt3 overexpression attenuates ROS production in oocyte. Exogenous Myc-Sirt3 mRNA or PBS was microinjected into fully-grown GV oocytes from diabetic mice, which were arrested at GV stage in M16 medium containing 2.5 μΜ milrinone for 20 hours to allow synthesis of new Myc-Sirt3 protein. Following in vitro-maturation, MII oocytes were stained by CM-H2DCFAD (green) to evaluate ROS levels. (A) Representative images of CM-H2DCFAD fluorescence in control, diabetic, diabetic+PBS and diabetic+Sirt3 oocytes. Scale bar: 25 μm. (B) Quantitative analysis of fluorescence intensity shown in B (n = 10 oocytes for each group). Data were expressed as mean ± SD from 3 independent experiments. *p < 0.05 vs control.
Sirt3 overexpression restores the acetylation levels of SOD2K68 in oocytes from diabetic mice
Our previous studies based on mutant screening have identified that lysine (K) 68 is likely to be a major acetylation site on SOD2 relating to ROS homeostasis in mouse oocytes.19 Moreover, Sirt3 has been demonstrated to be able to deacetylate SOD2K68 promoting its antioxidative activity.21,27 Since Sirt3 protein expression is reduced in diabetic oocytes (Fig. 2), we first examined whether the acetylation state of SOD2K68 in diabetic oocytes was disrupted accordingly. MII oocytes were labeled with anti-SOD2K68ac antibody and counterstained with Hoechst 33342 for chromosomes. Confocal microscopy and quantitative analysis (Fig. 4) revealed that the levels of SOD2K68 acetylation was increased about 2∼fold in diabetic oocytes compared with controls. Importantly, overexpression of exogenous Sirt3 was able to markedly lower the SOD2K68 acetylation in diabetic oocytes. Considering the impact of Sirt3 and SOD2 acetylation on ROS generation, we conclude that reduced Sirt3 expression in diabetic oocytes results in ROS accumulation probably through the hyperacetylation of SOD2K68.
Figure 4.
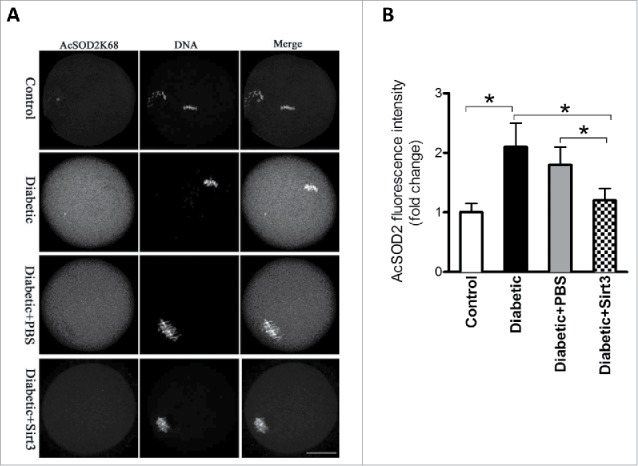
Sirt3 overexpression lowers the acetylation levels of SOD2K68 in oocytes from diabetic mice. Control (n = 77), diabetic (n = 71), diabetic+PBS (n = 80) and diabetic+Sirt3 (n = 76) MII oocytes were labeled with acetyl-SOD2K68 antibody and counterstained with Hoechst 33342 for chromosomes. (A) Representative images show the AcSOD2K68 signal and DNA in oocytes. Scale bar: 25 μm. (B) Quantitative analysis of fluorescence intensity shown in A. Data were expressed as mean ± SD from 3 independent experiments. *p < 0.05 vs control.
Deacetylation-mimetic mutant SOD2K68R ameliorates the oxidative stress in oocytes from diabetic mice
Given the hyperacetylation of SOD2 in oocytes exposed to maternal diabetes, we asked whether the non-acetylatable-mimetic mutant of SOD2 can partly prevent the oxidative damage in diabetic oocytes. For this purpose, mRNA for WT SOD2 or mutant SOD2 with lysine 68 changed to arginine (SOD2K68R) to mimic constitutively non-acetylated form as we described previously was injected into fully-grown oocytes from diabetic mice. Following in vitro-maturation, oocytes were stained by CM-H2DCFAD to evaluate ROS levels. As presented in Fig. 5, confocal microscopy and quantitative analysis showed that SOD2K68R significantly lowered the ROS levels compared with WT control. These results suggest that Sirt3-controlled SOD2K68 acetylation is directly linked to oxidative stress in diabetic oocytes.
Figure 5.
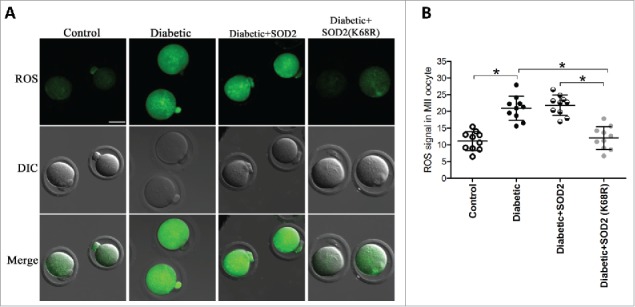
SOD2K68R partly prevents oxidative stress in oocytes from diabetic mice. SOD2 WT or SOD2K68R mutant mRNA was microinjected into fully-grown GV oocytes from diabetic mice, which were arrested at GV stage in M16 medium containing 2.5 μΜ milrinone for 20 hours to allow synthesis of new protein. Following in vitro-maturation, MII oocytes were stained with CM-H2DCFAD (green) to evaluate ROS levels. (A) Representative images of CM-H2DCFAD fluorescence in control, diabetic, diabetic+SOD2 and diabetic+SOD2 (K68R) oocytes. Scale bar: 25 μm. (B) Quantitative analysis of fluorescence intensity shown in A (n = 10 oocytes for each group). Data were expressed as mean ± SD from 3 independent experiments. *p < 0.05 vs control.
Discussion
Diabetic oocytes experience delayed meiotic maturation, abnormal cellular metabolism, as well as mitochondrial dysfunction.9,28 Mitochondrial functions have a dual effects on the intracellular redox state, via regeneration antioxidant systems which will decrease oxidative stress, and via ROS production which will increase oxidative stress.29,30 A few factors associated with mitochondrial metabolism, such as ATP, ROS, and pyruvate dehydrogenase complex, have been demonstrated have profound influences on the quality of mammalian oocytes.31-33 It should be noted that we detected the markedly increased ROS levels in oocytes from diabetic mice when compared with those from control mice (Fig. 1). This observation is consistent with previous findings that hyperglycemia can directly induce ROS generation.12 ROS produced primarily in the mitochondria as a byproduct of metabolism, and mitochondria are also a vulnerable target of ROS-induced oxidative damage.28,34 Meanwhile, other reports also showed that ROS mediates oxidative damage that may lead to mitochondrial dysfunction in mammalian oocytes.34,35 Particularly, mammalian oocytes are very sensitive to oxidative stress.36-39 Excessive ROS have deleterious effects on oocyte maturation, fertilization and even embryo development. Hence, we conclude that mitochondrial dysfunction in diabetic oocytes may be associated with the elevated ROS generation. It is believed that excessive ROS may be a cause of poor oocyte quality and metabolic alterations. However, the potential molecules controlling oocyte ROS levels remain unknown. Sirt3 is a member of Sirtuin family which predominantly resides in mitochondria and has been shown to be critical for the homeostasis of mitochondrial ROS.18 In the present study, we found that Sirt3 expression was diminished in diabetic oocytes (Fig. 2). Our previous data demonstrated that depletion of endogenous Sirt3 led to the increased ROS in oocyte.19 Through overexpression experiments, we here noted that elevating Sirt3 levels could attenuate ROS generation in diabetic oocytes (Fig. 3). Therefore, it is reasonable to speculate that Sirt3 play an important role in manipulating the ROS levels in mouse oocytes.
An intriguing finding from our work was that oocytes from diabetic mice lacking Sirt3 exhibited altered acetylation status of SOD2 (Fig. 4). Sirt3 plays a role in multiple metabolic processes by controlling mitochondrial pathway through the deacetylation of diverse targets. Among them, SOD2 was identified as a direct substrate of Sirt3. Sirt3 deacetylates critical lysine residues, i.e K53, K68, K89 and K122 on SOD2 and improve its catalytic activity, clearing cellular ROS.21,40,41 We found that K68 is a major target mediating Sirt3 control of ROS generation in mouse oocytes.19 Furthermore, overexpression of exogenous Sirt3 protein could lower the acetylation levels of SOD2K68 in diabetic oocytes (Fig. 4). Importantly, the forced expression of SOD2K68R apparently prevents the excessive ROS production in diabetic oocytes (Fig. 5). We cannot exclude the possibility that other targets controlled by Sirt3 participates in regulating redox state in oocytes. Based on these observations, we conclude that diabetic oocytes with Sirt3-deficiency lack the capacity to promote SOD2 activity in response to oxidative stress. Cumulatively, in this study, we have revealed a protective mechanism of Sirt3 against oxidative stress in diabetic oocytes via deacetylating SODK68. Considering the importance of mitochondrial ROS in determining oocyte quality, the present findings may provide a clue for improving oocyte in vitro-maturation system and assisted reproductive technologies.
Materials and methods
All chemicals and culture media were purchased from Sigma (St.Louis, MO, USA) unless stated otherwise. ICR mice were used in this study. All experiments were approved by the Animal Care and Use Committee of Nanjing Medical University and were performed in accordance with institutional guidelines.
Antibodies
Rabbit polyclonal anti-SIRT3 (Cat#:ab8667) and rabbit monoclonal anti-SOD2 (acetyl K68) (Cat#:ab137037) antibodies were purchased from Abcam (Cambridge, MA, USA); FITC-conjugated goat anti-rabbit IgG was purchased from Thermo Fisher Scientific (Rockford, IL, USA).
Generation of diabetic mice
To generate a diabetic model, female ICR mice 6-8 weeks of age were received a single injection of streptozotocin (STZ) at a dose of 190mg/kg. Four days after injection, a tail-blood sample was measured for glucose concentrations via a glucose analyzer. Mice with glucose levels greater than 300mg/dl were selected for use as diabetic model. Females without injection of STZ served as control.
Oocyte collection and culture
Female ICR mice were injected with 10 IU pregnant mare serum gonadotropin (PMSG) by intraperitoneal injection, and 46-48h later, cumulus-enclosed oocyte were obtained by manual rupturing of antral follicles. Cumulus cells were removed by repeatedly pipetting, fully-grown GV oocytes were obtained. For in vitro maturation, GV oocytes were cultured in M16 medium under mineral oil at 37℃ in 5% CO2 incubator. To collect ovulated MII oocytes, mice received an injection of 10 IU human chorionic gonadotropin (hCG) 46-48h after PMSG priming. Oocytes were recovered from the oviduct ampullae after 13.5h of hCG, and cumulus cells were removed by brief incubation in 1mg/ml hyaluronidase.
Plasmid construction and mRNA synthsis
Total RNA from mouse oocyte was extracted using the Arcturus PicoPure RNA Isolation Kit (Applied Biosystems, Foster City, CA, USA), and the cDNA was generated with QIAquick PCR Purification Kit (Qiagen, Düsseldorf, Germany). The primer used to amplify the CDS sequence of Sirt3, SOD2 and mutant are as follows:Sirt3
forward primer:5′–GGGGGCCGGCCGGTGGAGGAAGCAGTGAGAA–3′
reverse primer:5′–GGGGGCGCGCCCAGGTGAAGAAGCCATAGTC–3′
SOD2
forward primer:5′–GGGGGCCGGCCGAGAGCAGCGGTCGTGTA–3′
reverse primer:5′–GGGGGCGCGCCATGTGGCCGTGAGTGAG–3′
SOD2(K68R)
forward primer:5′–AACGCCACCGAGGAGAGGTACCACGAGGCTCTG–3′
reverse primer:5′–CAGAGCCTCGTGGTACCTCTCCTCGGTGGCGTT–3′
PCR products were purified, digested with FseI and AscI (NEB Inc., MA, USA), and then cloned into the PCS2+ vector encoding an N-terminal Myc tag. The PCS2+ vector encoding the SOD2 substitution mutant K68R was generated with the use of a QuickChange site-directed mutagenesis Kit (Stratagene). For mRNA synthesis, the PCS2+ plasmid were linearized by NotI. Capped cRNA were made using in vitro transcription with SP6 mMseeage mMachine (Ambion, Austin, TX, USA), and then purified by RNeasy Micro Kit (Qiagen). Synthesized RNA was portioned into aliquots and stored at −80℃.
Overexpression experiment
10pl mRNA solution (10 ng/μL) was microinjected into the cytoplasm of fully-grown GV oocyte with a Narishige microinjector. The same amount of RNase-free PBS was injected as control. To facilitate the mRNA translation, oocytes were arrested at GV stage in M16 medium containing 2.5 μΜ milrinone for 20 hours. After 3 washes, oocytes were cultured in milrinone-free M16 medium for further experiment.
Western blotting
A pool of 100 oocytes per sample was lysed in Laemmli sample buffer with protease inhibitor and heated for 5 min at 100℃. The denatured Proteins were separated by 10% SDS-PAGE and then electrically transferred to PVDF membranes. Membranes were blocked in PBST (PBS containing 0.1% Tween 20) with 5% low-fat dry milk for 1h. Then the membrane was incubated overnight at 4℃ with rabbit anti-Sirt3 antibody (1:500). After 3 washes in PBST, membranes were incubated with HRP-conjugated secondary antibodies for 1h at room temperature. After 3 washes, the protein bands were visualized by an ECL Plus Western Blotting Detection System (GE Healthcare, Little Chalfont, UK). The membranes were then washed in stripping buffer and re-probed with anti-tubulin antibody (1:5000) for loading control.
Measurement of intracellular ROS
To detect the quantity of ROS production in living oocytes, we used CM-H2DCFDA (Life Technologies, Invitrogen TM, Cat#:C6827). CM-H2DCFDA was prepared in DMSO before loading. Oocytes were incubated with 5μΜ CM-H2DCFDA for 30 min at 37℃ in 5% CO2 incubator. Then oocytes were washed 3 times and loaded on a slide with a microdrop of medium. Florescence intensity of each oocyte was detected under a Zeiss Laser Scanning Confocal Microscope (LSM 710, Zeiss, Germany) with an excitation wavelength of 488nm and an emission wavelength of 585nm.
Immunofluorescence
For staining of AcSOD2K68, oocytes were fixed in 4% paraformaldehyde for 30 min and permeabilized in 0.5% Triton X-100 for 20 min at room temperature. After blocking with 1% BSA-supplemented PBS for 1h, oocytes were incubated with rabbit monoclonal anti-SOD2K68ac antibody (1:300) at 4℃ overnight. After 3 washes in PBS containing 0.1% Tween 20 and 0.01% Triton X-100, oocytes were labeled with FITC-conjugated goat anti-rabbit secondary antibody for 1h at room temperature. Chromosomes were stained with Hoechest 33342 for 10 min. After 3 washes, oocytes were mounted on antifade medium (Vectashield, Burlingame, CA, USA), then examined under a Laser Scanning Confocal Microscope equipped with the 40 × objectives. Fluorescence intensity was quantified using Image J softwaer (NIH,USA).
Statistical analysis
Date are presented as mean ± SD, unless otherwise indicated. Differences between 2 groups were analyzed by Student,s t test. Multiple comparisons between more than 2 groups were analyzed by 1-way ANOVA test using Prism 5.0. P<0.05 was considered to be significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Natural Science Foundation (31571543 to QW and 31401227 to LG) of China, State Key Laboratory of Reproductive Medicine (SKLRM-KA201606), Jiangsu Entrepreneurship and Innovation Award (QW).
References
- [1].Colton SA, Pieper GM, Downs SM. Altered meiotic regulation in oocytes from diabetic mice. Biol Reprod 2002; 67:220-31; PMID:12080021; https://doi.org/ 10.1095/biolreprod67.1.220 [DOI] [PubMed] [Google Scholar]
- [2].Rosenn B, Miodovnik M, Combs CA, Khoury J, Siddiqi TA. Glycemic thresholds for spontaneous abortion and congenital malformations in insulin-dependent diabetes mellitus. Obstet Gynecol 1994; 84:515-20; PMID:8090386 [PubMed] [Google Scholar]
- [3].Chang AS, Dale AN, Moley KH. Maternal diabetes adversely affects preovulatory oocyte maturation, development, and granulosa cell apoptosis. Endocrinology 2005; 146:2445-53; PMID:15718275; https://doi.org/ 10.1210/en.2004-1472 [DOI] [PubMed] [Google Scholar]
- [4].Wyman A, Pinto AB, Sheridan R, Moley KH. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology 2008; 149:466-9; PMID:18039778; https://doi.org/ 10.1210/en.2007-1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Diamond MP, Moley KH, Pellicer A, Vaughn WK, DeCherney AH. Effects of streptozotocin- and alloxan-induced diabetes mellitus on mouse follicular and early embryo development. J Reprod Fertil 1989; 86:1-10; PMID:2526873; https://doi.org/ 10.1530/jrf.0.0860001 [DOI] [PubMed] [Google Scholar]
- [6].Veselá J, Rehák P, Baran V, Koppel J. Effects of healthy pseudopregnant milieu on development of two-cell subdiabetic mouse embryos. J Reprod Fertil 1994; 100:561-5; PMID:8021877; https://doi.org/ 10.1530/jrf.0.1000561 [DOI] [PubMed] [Google Scholar]
- [7].Moley KH, Vaughn WK, DeCherney AH, Diamond MP. Effect of diabetes mellitus on mouse pre-implantation embryo development. J Reprod Fertil 1991; 93:325-32; PMID:1787451; https://doi.org/ 10.1530/jrf.0.0930325 [DOI] [PubMed] [Google Scholar]
- [8].Ratchford A, Chang A, Chi M, Sheridan R, Moley K. Maternal diabetes adversely affects AMP-activated protein kinase activity and cellular metabolism in murine oocytes. Am J Physiol Endocrinol Metab 2007; 293:E1198-206; PMID:17684106; https://doi.org/ 10.1152/ajpendo.00097.2007 [DOI] [PubMed] [Google Scholar]
- [9].Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol 2009; 23:1603-12; PMID:19574447; https://doi.org/ 10.1210/me.2009-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005; 120:483-95; PMID:15734681; https://doi.org/ 10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- [11].Poston L, Igosheva N, Mistry HD, Seed PT, Shennan AH, Rana S, Karumanchi SA, Chappell LC. Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am J Clin Nutr 2011; 94:1980S-5S; PMID:21613560; https://doi.org/ 10.3945/ajcn.110.001156 [DOI] [PubMed] [Google Scholar]
- [12].Witczak M, Ferenc T, Gulczynska E, Nowakowska D, Lopaczynska D, Wilczynski J. Elevated frequencies of micronuclei in pregnant women with type 1 diabetes mellitus and in their newborns. Mutat Res Genet Toxicol Environ Mutagen 2014; 763:12-7; PMID:24561380; https://doi.org/ 10.1016/j.mrgentox.2014.02.002 [DOI] [PubMed] [Google Scholar]
- [13].Ornoy A, Livshitz A, Ergaz Z, Stodgell CJ, Miller RK. Hyperglycemia, hypoxia and their combination exert oxidative stress and changes in antioxidant gene expression: Studies on cultured rat embryos. Birth Defects Res B Dev Reprod Toxicol 2011; 92:231-9; PMID:21702078; https://doi.org/ 10.1002/bdrb.20313 [DOI] [PubMed] [Google Scholar]
- [14].Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, et al.. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008; 44:280-7; PMID:18339123; https://doi.org/ 10.1111/j.1600-079X.2007.00524.x [DOI] [PubMed] [Google Scholar]
- [15].Zhang T, Zhou Y, Wang HH, Ma XS, Qian WP, Shen W, Schatten H, Sun QY. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging (Albany NY) 2016; 8:685-96; PMID:26974211; https://doi.org/ 10.18632/aging.100911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sultana MR, Bagul PK, Katare PB, Anwar Mohammed S, Padiya R, Banerjee SK. Garlic activates SIRT-3 to prevent cardiac oxidative stress and mitochondrial dysfunction in diabetes. Life Sci 2016; 164:42-51; PMID:27590611; https://doi.org/ 10.1016/j.lfs.2016.08.030 [DOI] [PubMed] [Google Scholar]
- [17].Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010; 143:802-12; PMID:21094524; https://doi.org/ 10.1016/j.cell.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bause AS, Haigis MC. SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol 2013; 48:634-9; PMID:22964489; https://doi.org/ 10.1016/j.exger.2012.08.007 [DOI] [PubMed] [Google Scholar]
- [19].Zhang L, Han L, Ma R, Hou X, Yu Y, Sun S, Xu Y, Schedl T, Moley KH, Wang Q. Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle 2015; 14:2959-68; PMID:25790176; https://doi.org/ 10.1080/15384101.2015.1026517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kawamura Y, U Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest 2010; 120:2817-28; PMID:20644252; https://doi.org/ 10.1172/JCI42020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep 2011; 12:534-41; PMID:21566644; https://doi.org/ 10.1038/embor.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xiong Y, Guan KL. Mechanistic insights into the regulation of metabolic enzymes by acetylation. J Cell Biol 2012; 198:155-64; PMID:22826120; https://doi.org/ 10.1083/jcb.201202056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 2017; 16(1):4-16; PMID:27686535; https://doi.org/ 10.1111/acel.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng PP, Xia JJ, Wang HL, Chen JB, Wang FY, Zhang Y, Huang X, Zhang QJ, Qi ZQ. Islet transplantation reverses the effects of maternal diabetes on mouse oocytes. Reproduction 2011; 141:417-24; PMID:21273367; https://doi.org/ 10.1530/REP-10-0370 [DOI] [PubMed] [Google Scholar]
- [25].Wang Q, Moley KH. Maternal diabetes and oocyte quality. Mitochondrion 2010; 10:403-10; PMID:20226883; https://doi.org/ 10.1016/j.mito.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D. SIRT3 reverses aging-associated degeneration. Cell Rep 2013; 3:319-27; PMID:23375372; https://doi.org/ 10.1016/j.celrep.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metabolism 2010; 12:662-7; PMID:21109198; https://doi.org/ 10.1016/j.cmet.2010.11.015 [DOI] [PubMed] [Google Scholar]
- [28].Wang Q, Frolova A, Purcell S, Adastra K, Schoeller E, Chi MM, Schedl T, Moley KH. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One 2010; 28:e15901; https://doi.org/ 10.1371/journal.pone.0015901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol 2009; 20:346-53; PMID:19530278; https://doi.org/ 10.1016/j.semcdb.2008.12.013 [DOI] [PubMed] [Google Scholar]
- [30].Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One 2010; 5:e10074; PMID:20404917; https://doi.org/ 10.1371/journal.pone.0010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Choi WJ, Banerjee J, Falcone T, Bena J, Agarwal A, Sharma RK. Oxidative stress and tumor necrosis factor-alpha-induced alterations in metaphase II mouse oocyte spindle structure. Fertil Steril 2007; 88:1220-31; PMID:17601599; https://doi.org/ 10.1016/j.fertnstert.2007.02.067 [DOI] [PubMed] [Google Scholar]
- [32].Johnson MT, Freeman EA, Gardner DK, Hunt PA. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod 2007; 77:2-8; PMID:17314311; https://doi.org/ 10.1095/biolreprod.106.059899 [DOI] [PubMed] [Google Scholar]
- [33].Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res 2006; 16:841-50; PMID:16983401; https://doi.org/ 10.1038/sj.cr.7310095 [DOI] [PubMed] [Google Scholar]
- [34].Wu J, Jin Z, Yan LJ. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol 2016; 11:51-9; PMID:27888691; https://doi.org/ 10.1016/j.redox.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod 2000; 62:1745-53; PMID:10819779; https://doi.org/ 10.1095/biolreprod62.6.1745 [DOI] [PubMed] [Google Scholar]
- [36].Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, Thornton J, Agarwal A. Differential growth of human embryos in vitro: Role of reactive oxygen species. Fertil Steril 2004; 82:593-600; PMID:15374701; https://doi.org/ 10.1016/j.fertnstert.2004.02.121 [DOI] [PubMed] [Google Scholar]
- [37].Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005; 3:28; PMID:16018814; https://doi.org/ 10.1186/1477-7827-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early embryo development. Reproduction 2002; 123:479-86; PMID:11914110; https://doi.org/ 10.1530/rep.0.1230479 [DOI] [PubMed] [Google Scholar]
- [39].Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod Update 1998; 13:998-1002; https://doi.org/ 10.1093/humrep/13.4.998 [DOI] [PubMed] [Google Scholar]
- [40].Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, et al.. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 2010; 40:893-904; PMID:21172655; https://doi.org/ 10.1016/j.molcel.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: Metabolism, cell death and aging. Biochem J 2012; 444:1-10; PMID:22533670; https://doi.org/ 10.1042/BJ20120030 [DOI] [PubMed] [Google Scholar]


