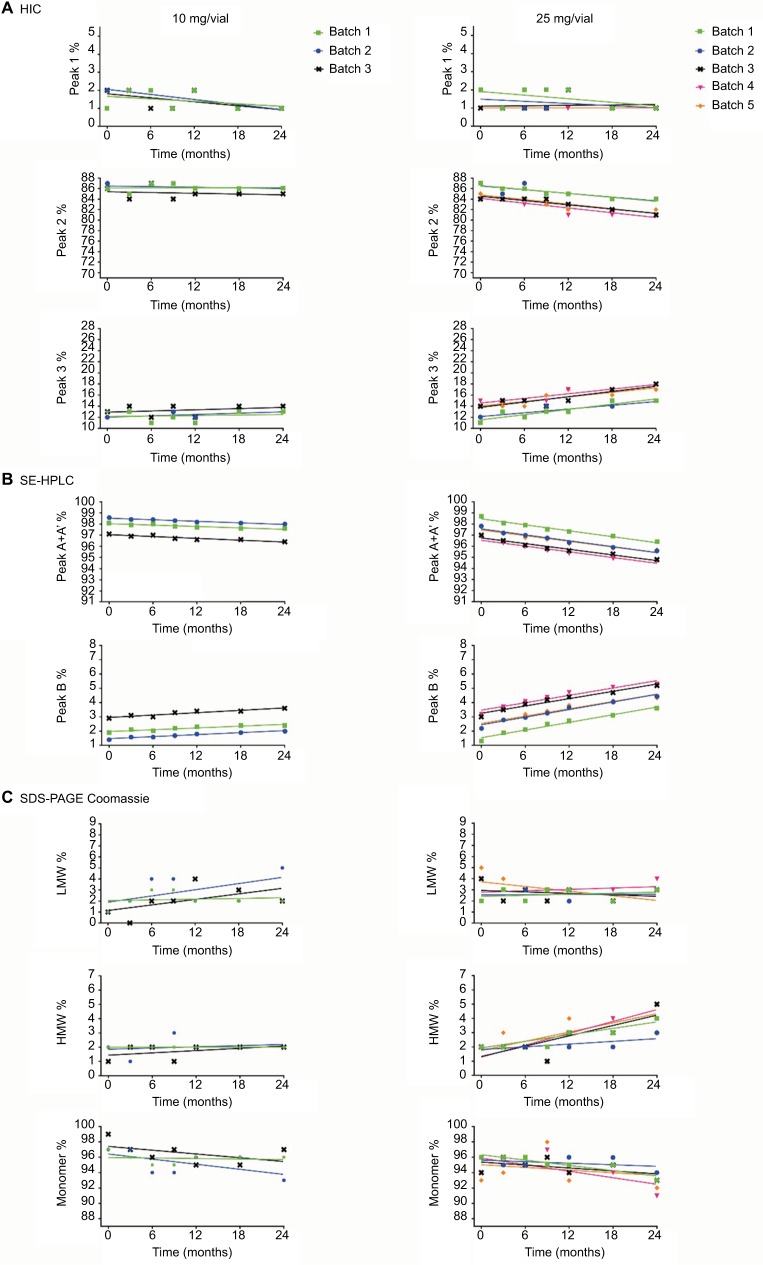
Figure 1.
Stability data for the quantitative stability-indicating assays (A) HIC, (B) SE-HPLC, and (C) SDS-PAGE Coomassie for etanercept 10 mg/vial (n=3 batches) and 25 mg/vial Lyo DP (n=5 batches) at the alternative storage condition of 25°C±2°C through to 24 months.
Abbreviations: HIC, hydrophobic interaction chromatography; SE-HPLC, size exclusion high-performance liquid chromatography; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; LMW, low molecular weight; HMW, high molecular weight; Lyo, lyophilized powder; DP, drug product.