Abstract
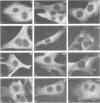
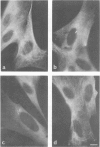
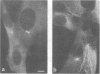
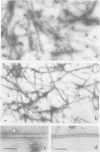
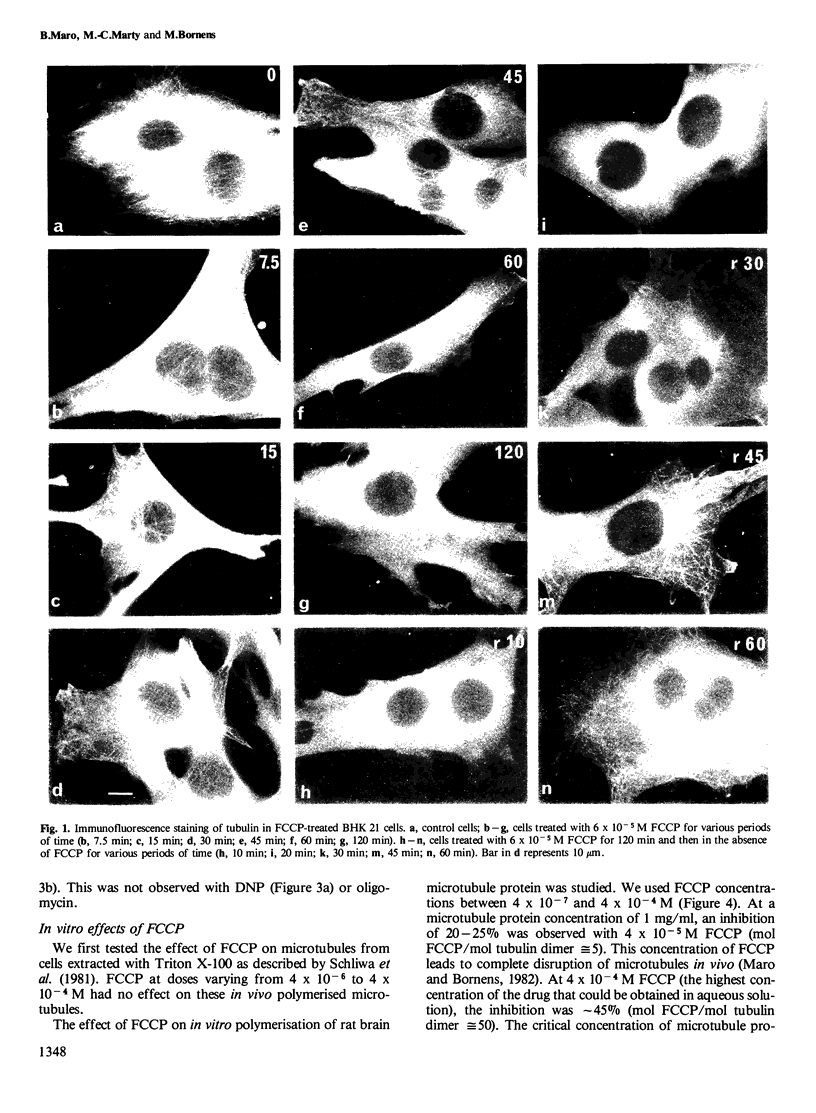
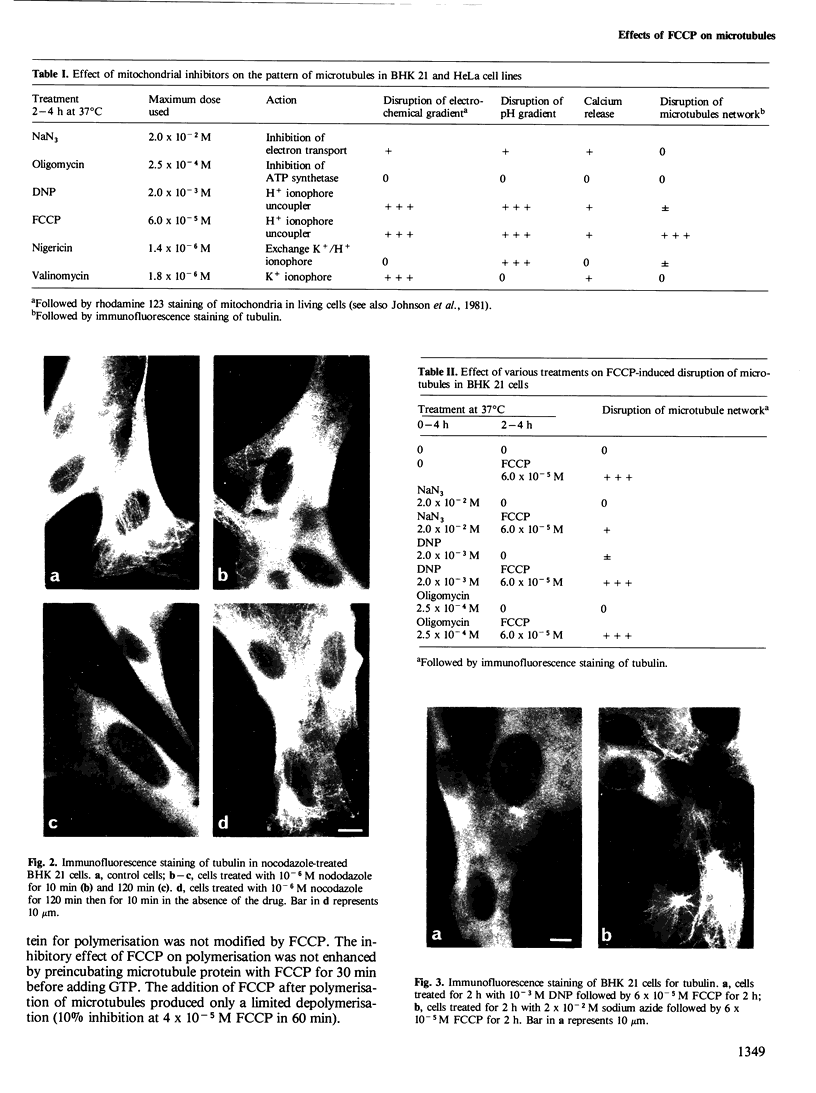
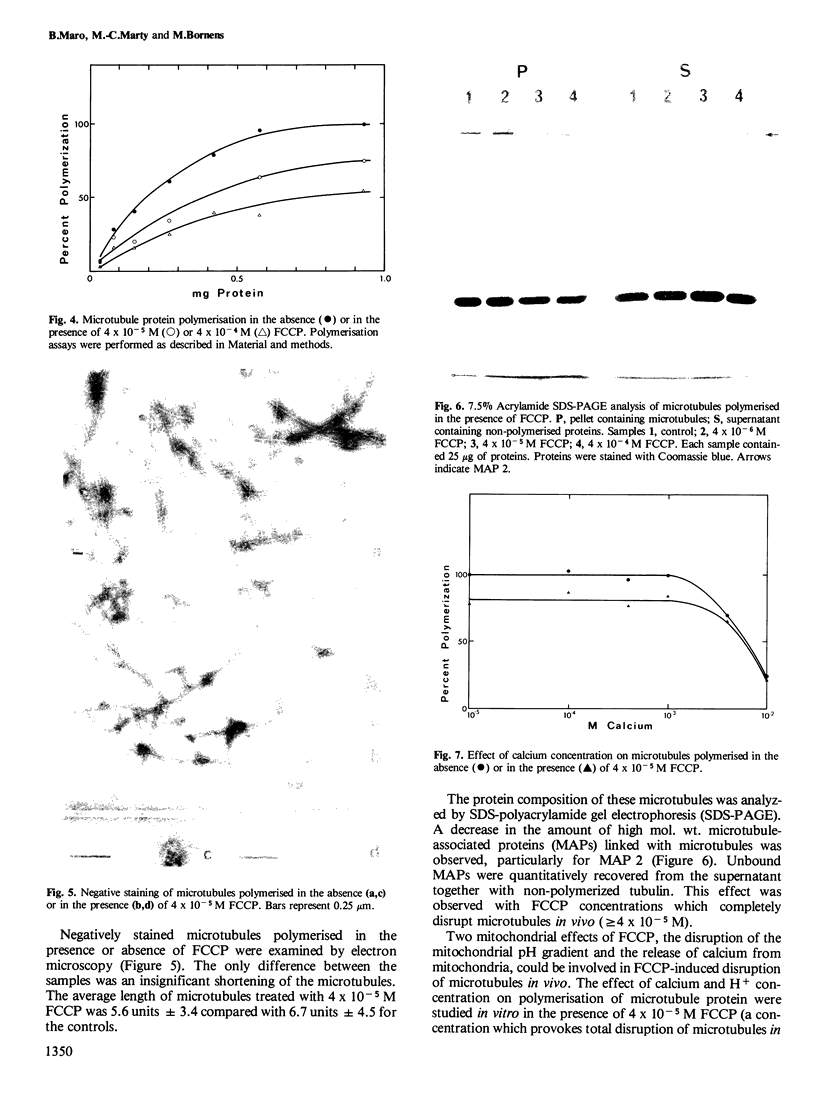
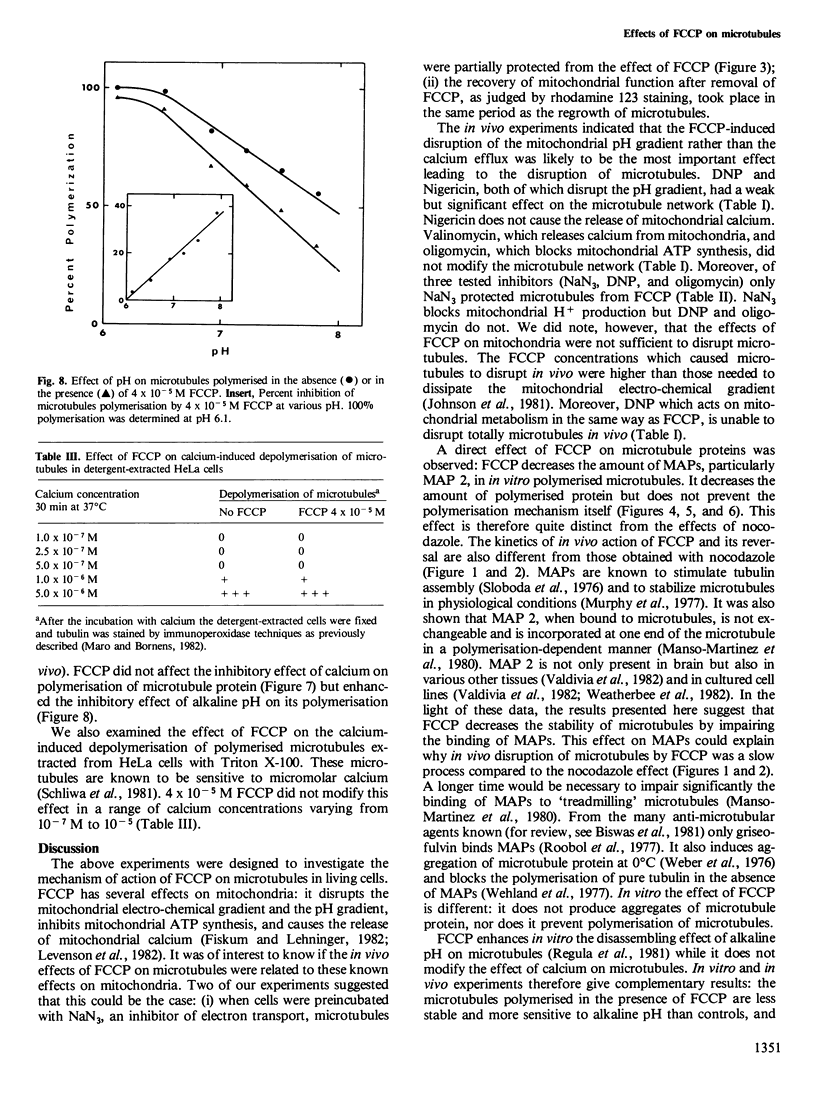
FCCP (carbonylcyanide-p-trifluoromethoxyphenylhydrazone), a potent uncoupler of oxidative phosphorylation, induces the complete disruption of cellular microtubules. A further analysis of this effect on BHK21 cells has shown that a decrease in the number of microtubules can be observed 15 min after adding FCCP and there is complete disruption after 60 min. Regrowth of microtubules was initiated 30 min after removal of FCCP, in marked contrast with the rapid reversion observed when microtubules are disrupted by nocodazole. A similar delay was required for the recovery of mitochondrial function as assessed by rhodamine 123 labelling. The effect of FCCP on microtubules was partially inhibited by preincubation of the cells with NaN3, suggesting that FCCP acts on microtubules through mitochondria. FCCP did not depolymerize microtubules of cells permeabilized with Triton X-100. In vitro polymerisation of microtubule protein was only slightly diminished by concentrations of FCCP which provoke complete disassembly in vivo. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the microtubules polymerized in vitro in the presence of FCCP showed a reduced amount of high mol. wt. proteins, mainly MAP 2, associated with them. In an attempt to reproduce the mitochondrial effects of FCCP in vitro, we checked the effects of alkaline pH and calcium on microtubule protein polymerization in the presence of FCCP. FCCP did not influence the calcium inhibitory effect but did significantly increase the inhibitory effect of alkaline pH. We conclude that FCCP could depolymerise microtubules in vivo through a dual operation: increasing the intracellular pH by the disruption of the mitochondrial H+ gradient and decreasing the stability of microtubules by impairing the binding of microtubule-associated proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball E. H., Singer S. J. Mitochondria are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1982 Jan;79(1):123–126. doi: 10.1073/pnas.79.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier-Valentin F., Rousset B. Interaction of tubulin with rat liver mitochondria. J Biol Chem. 1982 Jun 25;257(12):7092–7099. [PubMed] [Google Scholar]
- Biswas B. B., Banerjee A. C., Bhattacharyya B. Tubulin and the microtubule system in cellular growth and development. Subcell Biochem. 1981;8:123–183. doi: 10.1007/978-1-4615-7951-9_3. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A. Organelle-cytoskeleton relationships in fibroblasts: mitochondria, Golgi apparatus, and endoplasmic reticulum in phases of movement and growth. Eur J Cell Biol. 1982 Apr;27(1):47–54. [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebeke J., Van Nijen G., De Brabander M. Interaction of oncodazole (R 17934), a new antitumoral drug, with rat brain tubulin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):319–324. doi: 10.1016/0006-291x(76)90524-6. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Guilbert B., Bornens M., Avrameas S., Whalen R., Pantaloni D. Detection of tubulin and actin in various cell lines by an immunoperoxidase technique. J Histochem Cytochem. 1978 Nov;26(11):934–947. doi: 10.1177/26.11.363933. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levenson R., Macara I. G., Smith R. L., Cantley L., Housman D. Role of mitochondrial membrane potential in the regulation of murine erythroleukemia cell differentiation. Cell. 1982 Apr;28(4):855–863. doi: 10.1016/0092-8674(82)90064-2. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Feramisco J. R. Disruption of the in vivo distribution of the intermediate filaments in fibroblasts through the microinjection of a specific monoclonal antibody. Cell. 1981 Apr;24(1):185–193. doi: 10.1016/0092-8674(81)90514-6. [DOI] [PubMed] [Google Scholar]
- Manso-Martínez R., Villasante A., Avila J. Incorporation of the high-molecular-weight microtubule-associated protein 2 (MAP2) into microtubules at steady state in vitro. Eur J Biochem. 1980 Apr;105(2):307–313. doi: 10.1111/j.1432-1033.1980.tb04502.x. [DOI] [PubMed] [Google Scholar]
- Maro B., Bornens M. Reorganization of HeLa cell cytoskeleton induced by an uncoupler of oxidative phosphorylation. Nature. 1982 Jan 28;295(5847):334–336. doi: 10.1038/295334a0. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Vallee R. B., Borisy G. G. Identity and polymerization-stimulatory activity of the nontubulin proteins associated with microtubules. Biochemistry. 1977 Jun 14;16(12):2598–2605. doi: 10.1021/bi00631a004. [DOI] [PubMed] [Google Scholar]
- Regula C. S., Pfeiffer J. R., Berlin R. D. Microtubule assembly and disassembly at alkaline pH. J Cell Biol. 1981 Apr;89(1):45–53. doi: 10.1083/jcb.89.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol A., Gull K., Pogson I. Evidence that griseofulvin binds to a microtubule associated protein. FEBS Lett. 1977 Mar 15;75(1):149–153. doi: 10.1016/0014-5793(77)80073-2. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Euteneuer U., Bulinski J. C., Izant J. G. Calcium lability of cytoplasmic microtubules and its modulation by microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1037–1041. doi: 10.1073/pnas.78.2.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda R. D., Dentler W. L., Rosenbaum J. L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976 Oct 5;15(20):4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- Smith D. S., Järlfors U., Cayer M. L. Structural cross-bridges between microtubules and mitochondria in central axons of an insect (Periplaneta americana). J Cell Sci. 1977;27:255–272. doi: 10.1242/jcs.27.1.255. [DOI] [PubMed] [Google Scholar]
- Valdivia M. M., Avila J., Coll J., Colaço C., Sandoval I. V. Quantitation and characterization of the microtubule associated MAP2 in porcine tissues and its isolation from porcine (PK15) and human (HeLa) cell lines. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1241–1249. doi: 10.1016/0006-291x(82)90920-2. [DOI] [PubMed] [Google Scholar]
- Weatherbee J. A., Sherline P., Mascardo R. N., Izant J. G., Luftig R. B., Weihing R. R. Microtubule-associated proteins of HeLa cells: heat stability of the 200,000 mol wt HeLa MAPs and detection of the presence of MAP-2 in HeLa cell extracts and cycled microtubules. J Cell Biol. 1982 Jan;92(1):155–163. doi: 10.1083/jcb.92.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Wehland J., Herzog W. Griseofulvin interacts with microtubules both in vivo and in vitro. J Mol Biol. 1976 Apr 25;102(4):817–829. doi: 10.1016/0022-2836(76)90293-x. [DOI] [PubMed] [Google Scholar]
- Wehland J., Herzog W., Weber K. Interaction of griseofulvin with microtubules, microtubule protein and tubulin. J Mol Biol. 1977 Apr 15;111(3):329–342. doi: 10.1016/s0022-2836(77)80055-7. [DOI] [PubMed] [Google Scholar]