Figure 1.
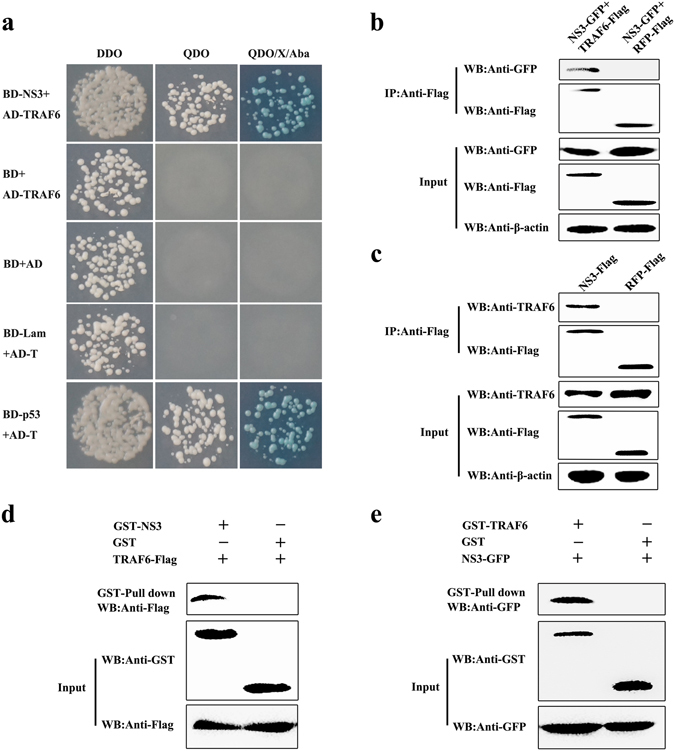
Interaction of NS3 with TRAF6. (a) Interaction of NS3 with TRAF6 in a yeast two-hybrid system. The yeast strain Y2HGold was cotransformed with the prey plasmid AD-TRAF6 and the bait plasmid BD or BD-NS3. Cotransformation with BD/AD, BD-Lamin/AD-T, and BD-p53/AD-T were used as a blank, negative and positive controls, respectively. (b) Exogenous co-IP analysis of NS3 and TRAF6 in PAMs. Cells were co-transfected with plasmids NS3-GFP and TRAF6-Flag. PAMs co-transfected with NS3-GFP and RFP-Flag were used as negative controls. A quarter of the cell extract was subjected to the input assay to assess β-actin, Flag-fusion, and GFP-fusion protein levels. The rest of the extract was subjected to IP assay. Western blot detected proteins with a mouse anti-GFP mAb and a rabbit anti-Flag pAb. (c) Endogenous co-IP analysis of NS3 and TRAF6 in PAMs. Cells were transfected with plasmid NS3-Flag, and RFP-Flag-transfected PAMs were used as negative controls. The input assay was performed using a quarter of the cell extract to assess β-actin, Flag fusion protein, and TRAF6 levels. The precipitated proteins were detected with a rabbit anti-TRAF6 pAb and a rabbit anti-Flag pAb. (d) GST-NS3 pull-down assay. The GST and GST-NS3 proteins expressed in Escherichia coli Rosetta (DE3) cells were immobilized on a glutathione agarose resin, followed by incubation of the resin with the cell lysates containing TRAF6-Flag protein. After washing, the bound proteins were detected by Western blot using a mouse anti-Flag mAb. The expression of input proteins (TRAF6-Flag, GST or GST-NS3) was confirmed by Western blot using a mouse anti-Flag mAb and a mouse anti-GST mAb, respectively. (e) GST-TRAF6 pull-down assay. The GST and GST-TRAF6 proteins expressed in Escherichia coli Rosetta (DE3) cells were immobilized on the glutathione-agarose resin. The resin conjugated with GST or GST-TRAF6 was incubated with the cell lysates containing NS3-GFP protein. After washing, the eluted proteins were detected by Western blot using a mouse anti-GFP mAb.
