Abstract
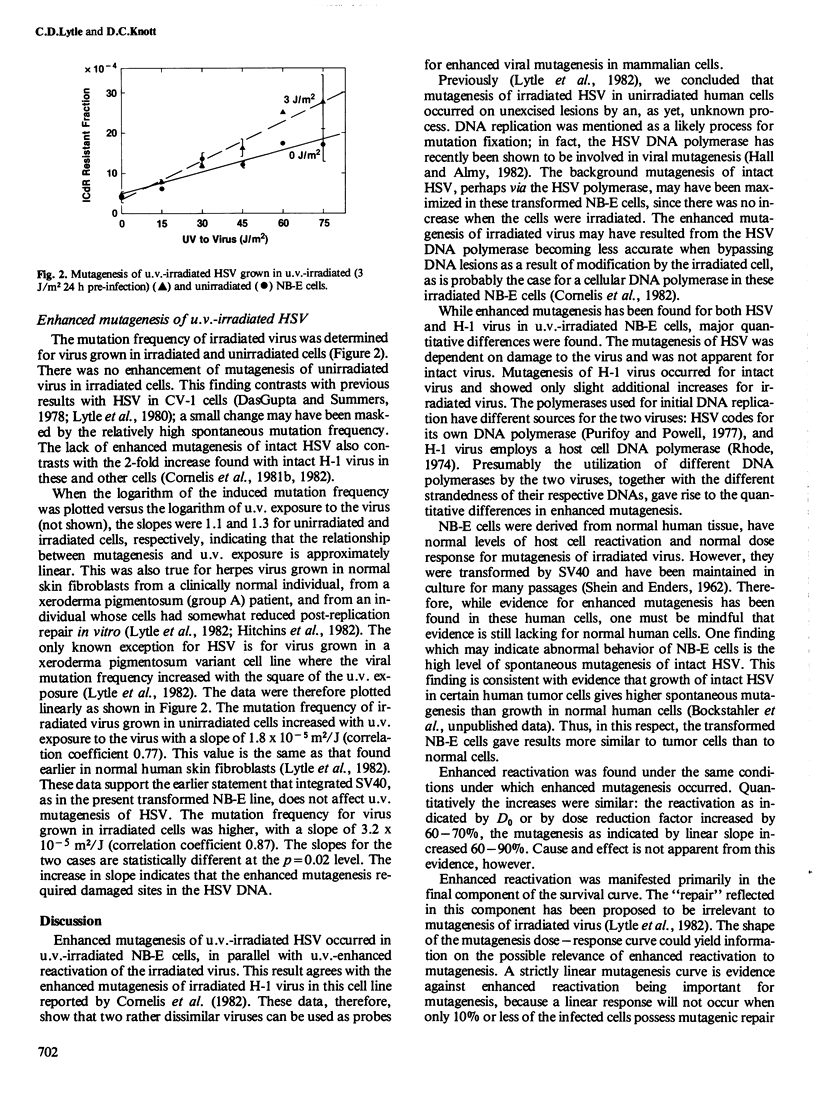
U.v. irradiation of human NB-E cells results in enhanced mutagenesis and enhanced reactivation of u.v.-irradiated H-1 virus grown in those cells ( Cornelis et al., 1982). This paper reports a similar study using herpes simplex virus (HSV) in NB-E cells. The mutation frequency of HSV (resistance of virus plaque formation to 40 micrograms/ml iododeoxycytidine ) increased approximately linearly with exposure of the virus to u.v. radiation. HSV grown in unirradiated cells gave a slope of 1.8 X 10(-5)m2/J, with 3.2 X 10(-5)m2/J for HSV grown in cells irradiated (3 J/m2) 24 h before infection. There was no evidence for mutagenesis of unirradiated virus by irradiated cells, as seen with H-1 virus. Enhanced reactivation of irradiated HSV in parallel cultures increased virus survival, manifested as a change in slope of the final component of the two-component survival curve from a D0 of 27 J/m2 in unirradiated cells to 45 J/m2 in irradiated cells. Thus, enhanced mutagenesis and enhanced reactivation occurred for irradiated HSV in NB-E cells. The difference in the enhanced mutagenesis of HSV (dependent on damaged DNA sites) and of H-1 virus (primarily independent of damaged DNA sites) is discussed in terms of differences in DNA polymerases.
Full text
PDF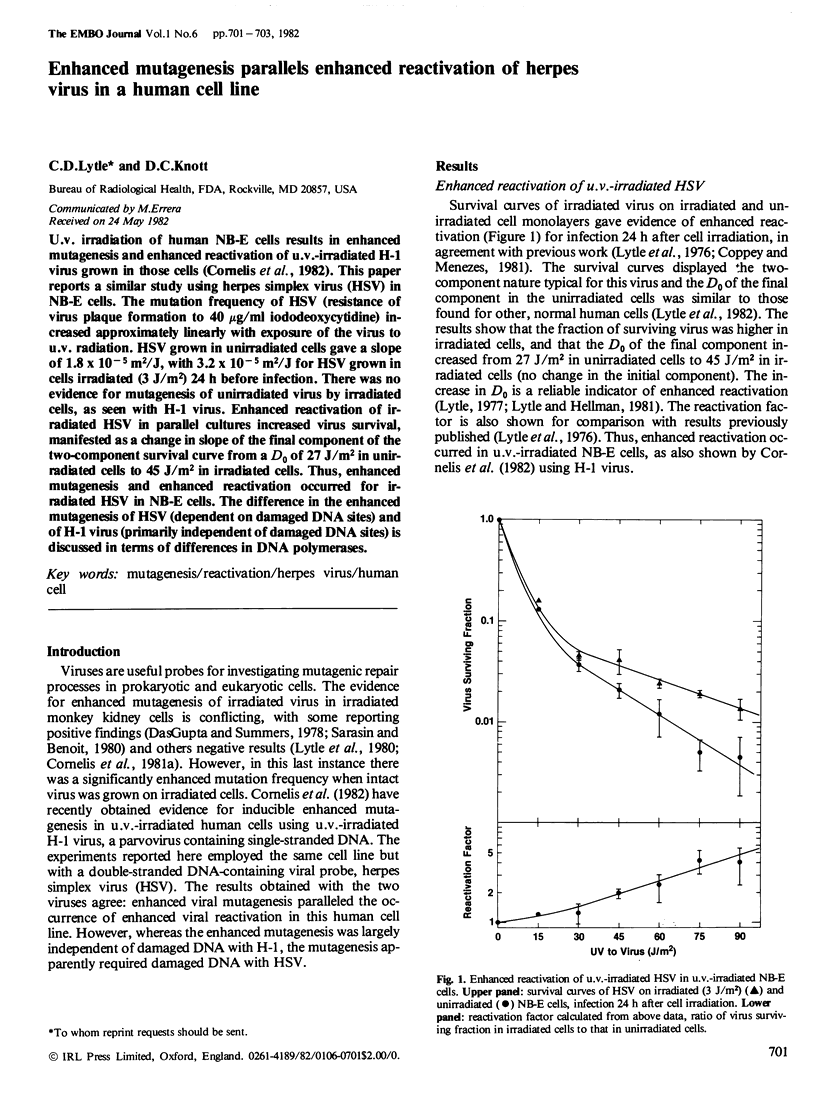

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coppey J., Menezes S. Enhanced reactivation of ultraviolet-damaged herpes virus in ultraviolet pretreated skin fibroblasts of cancer prone donors. Carcinogenesis. 1981;2(8):787–793. doi: 10.1093/carcin/2.8.787. [DOI] [PubMed] [Google Scholar]
- Cornelis J. J., Lupker J. H., Klein B., van der Eb A. J. The effect of cell irradiation on mutation in ultraviolet-irradiated and intact simian virus 40. Mutat Res. 1981 Jun;82(1):1–10. doi: 10.1016/0027-5107(81)90132-9. [DOI] [PubMed] [Google Scholar]
- Cornelis J. J., Su Z. Z., Rommelaere J. Direct and indirect effects of ultraviolet light on the mutagenesis of parvovirus H-1 in human cells. EMBO J. 1982;1(6):693–699. doi: 10.1002/j.1460-2075.1982.tb01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis J. J., Su Z. Z., Ward D. C., Rommelaere J. Indirect induction of mutagenesis of intact parvovirus H-1 in mammalian cells treated with UV light or with UV-irradiated H-1 or simian virus 40. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4480–4484. doi: 10.1073/pnas.78.7.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta U. B., Summers W. C. Ultraviolet reactivation of herpes simplex virus is mutagenic and inducible in mammlian cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2378–2381. doi: 10.1073/pnas.75.5.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. D., Almy R. E. Evidence for control of herpes simplex virus mutagenesis by the viral DNA polymerase. Virology. 1982 Jan 30;116(2):535–543. doi: 10.1016/0042-6822(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Day R. S., 3rd, Hellman K. B., Bockstahler L. E. Infection of UV-irradiated xeroderma pigmentosum fibroblasts by herpes simplex virus: study of capacity and Weigle reactivation. Mutat Res. 1976 Sep;36(3):257–264. doi: 10.1016/0027-5107(76)90235-9. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Goddard J. G., Lin C. H. Repair and mutagenesis of herpes simplex virus in UV-irradiated monkey cells. Mutat Res. 1980 Apr;70(2):139–149. doi: 10.1016/0027-5107(80)90153-0. [DOI] [PubMed] [Google Scholar]
- Lytle C. D. Host-cell reactivation in mammalian cells. I. Survival of ultra-violet-irradiated herpes virus in different cell-lines. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(4):329–337. doi: 10.1080/09553007114550451. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Nikaido O., Hitchins V. M., Jacobson E. D. Host cell reactivation by excision repair is error-free in human cells. Mutat Res. 1982 Jun;94(2):405–412. doi: 10.1016/0027-5107(82)90303-7. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. Herpes simplex virus DNA polymerase as the site of phosphonoacetate sensitivity: temperature-sensitive mutants. J Virol. 1977 Nov;24(2):470–477. doi: 10.1128/jvi.24.2.470-477.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. III. Factors affecting H-1 RF DNA synthesis. J Virol. 1974 Oct;14(4):791–801. doi: 10.1128/jvi.14.4.791-801.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A., Benoit A. Induction of an error-prone mode of DNA repair in UV-irradiated monkey kidney cells. Mutat Res. 1980 Mar;70(1):71–81. doi: 10.1016/0027-5107(80)90059-7. [DOI] [PubMed] [Google Scholar]


