Figure 2.
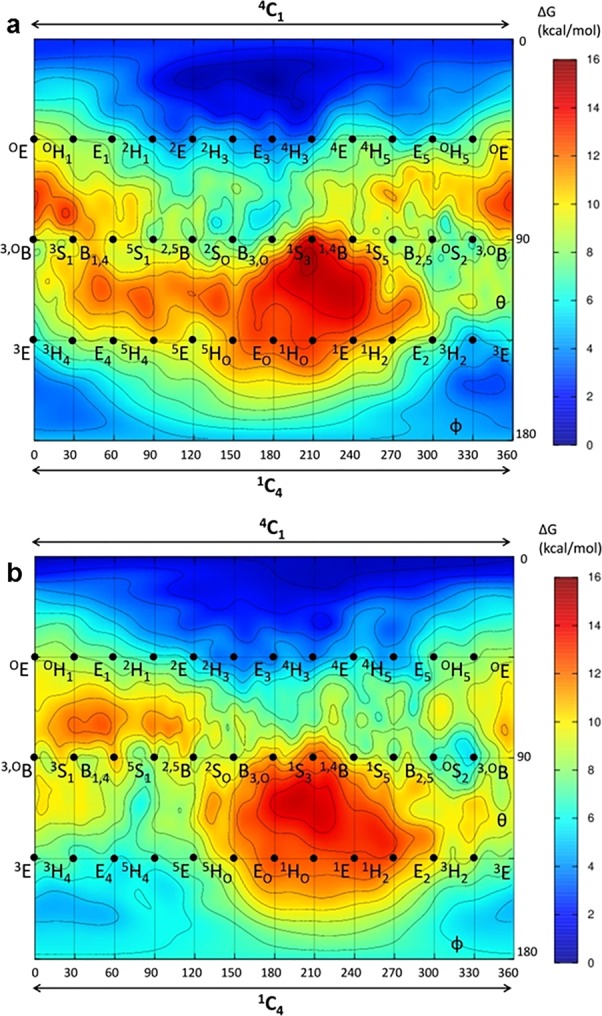
Conformational free energy landscapes of cyclosulfates 5 and 6. Cyclosulfates 5 (a) and 6 (b) adopt 4C1 ground state conformations, with a broad energy minimum extending toward 4H3. The x and y axes of each graph correspond to the φ and θ Cremer–Pople puckering coordinates (in degrees), respectively. Isolines are 1 kcal/mol.
