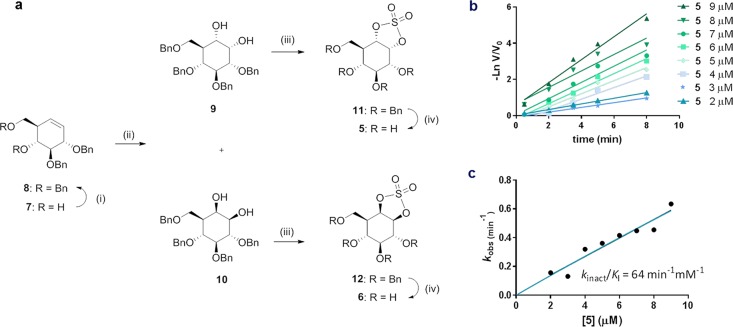
Figure 3.
Synthesis of cyclophellitol cyclosulfates 5 and 6, and inactivation of GAA by compound 5. (a) Compounds 5 and 6 were prepared from cyclohexene 11. Reagents and conditions: (i) BnBr, TBAI, NaH, DMF, rt, 18 h, 70%; (ii) RuCl3, NaIO4, EtOAc, ACN, 0 °C, 2 h (13, 39%; 14, 26%); (iii) (a) SOCl2, Et3N, DCM, 0 °C, (b) RuCl3, NaIO4, CCl4, ACN, 0 °C, 3 h (15, 59%; 16, 62%); (iv) H2, Pd/C, MeOH, rt, 18 h (5, 71%; 6, 72%). (b) Semilogarithmic plots of residual activity of GAA versus time at 9, 8, 7, 6, 5, 4, 3, and 2 μM α-cyclosulfate 5. (c) Plot of pseudo first order rate constants from panel c vs concentration of 5.