Abstract
Oxidative stress/damage resulting from exposure to cigarette smoke plays a critical role in the development of tobacco-caused diseases. Carbonyls and free radicals are two major classes of oxidants in tobacco smoke. There is little information on the combined delivery of these oxidants across different cigarette brands; thus, we set out to measure and compare their levels in mainstream smoke from popular US cigarettes. Mainstream smoke from 28 different cigarette brands produced by smoking (FTC protocol) was analyzed for five important, abundant carbonyls, and levels were compared to previously determined free radical for the same brands. Overall, there were large variations (3- to 6-fold) in carbonyl levels across brands with total carbonyl levels ranging from 275 to 804 μg/cigarette, which persisted even after adjusting for ventilation. Individual carbonyl levels were highly correlated with each other (r2: 0.40–0.95, P<0.003) except for formaldehyde. Both gas-phase (r2: 0.37, P=0.006) and particulate-phase (r2: 0.27, P=0.005) free radicals were correlated to total carbonyl content; however, this correlation disappeared after adjusting for ventilation. These data show that overall oxidant production varies widely by cigarette brand and the resulting difference in oxidant burden could potentially lead to differences in disease risk.
Keywords: Acetaldehyde, formaldehyde, crotonaldehyde, methyl ethyl ketone, propionaldehyde, oxidants, tobacco smoke
Graphical Abstract
1. Introduction
Cigarette smoking is the number one cause of preventable death worldwide, causing one of every five deaths in the United States (U.S.) each year (Danaei et al., 2009; U.S. Department of Health and Human Services, 2014; Xu et al., 2015). It leads to lung cancer (Doll and Hill, 1950; Wynder and Graham, 1950), chronic obstructive pulmonary disease (COPD) (Burney et al., 2014; Zaher et al., 2004), cardiovascular and many other diseases (Krupski, 1991; U.S. Department of Health and Human Services, 2014). Oxidative stress is thought to play a major role in the development of many of these tobacco-caused diseases (Church and Pryor, 1985; Pryor, 1997); thus, it is important to better understand the total oxidant burden resulting from cigarette smoke in order to gain a better assessment of a smoker’s potential oxidative risk.
While carbonyls are most known for their toxic and carcinogenic effects (U.S. Food and Drug Administration, 2012), carbonyls are also a major class of powerful oxidants, depleting glutathione (Wooten et al., 2006), forming adducts with DNA bases (Halliwell and Gutteridge, 2015), and producing free radicals when metabolized (Kundu et al., 2007). Together, these effects have linked carbonyls to a significant number of smoking-related diseases (U.S. Food and Drug Administration, 2012). Previous reports on carbonyls in cigarette smoke show their levels vary by brand (Bodnar et al., 2012; Counts et al., 2004; Counts et al., 2005; Ding et al., 2015; Hammond and O’Conner, 2008; Marcilla et al., 2012; Roemer et al., 2003). Potential sources of variation between cigarette brands include differences in ventilation, tobacco type, filter design, additives, and paper porosity as well as a smoker’s unique puff profile (Baker et al., 2004a, b; Chen et al., 2014; Dittrich et al., 2014; Hoffmann et al., 1995; Roemer et al., 2012; Seeman et al., 2003). While carbonyl production is known to differ by brand, little is known as to how this variation may compare to the other major class of oxidants, free radicals. Thus, we sought to determine the variation in the levels of five important and abundant tobacco smoke carbonyls (formaldehyde, acetaldehyde, propionaldehyde, crotonaldehyde, and methyl ethyl ketone (MEK)) and compare their levels to those of free radicals. Since both classes of oxidants are produced by the incomplete combustion and pyrolysis of the tobacco in the cigarette (U.S. Department of Health and Human Services, 2014), we hypothesize that these levels will vary similarly amongst brand/type. Altogether, this information can be used to better understand the total oxidant burden of cigarette smoke. To this end, we recently developed standardized methodology to analyze both the highly reactive gas-phase free radical content and the stable particulate-phase free radical content of cigarette smoke and applied the method to quantify the free radical levels in 27 brands of popular US cigarettes for the first time (Goel et al., 2017). In the current paper, we report on the levels of carbonyls in the same 27 cigarette brands smoked under identical conditions (FTC protocol) and use these results to determine the relationship of these two oxidant classes across cigarette brands.
2. Materials and Methods
2.1 Cigarettes
Twenty-seven cigarette brands (all king-sized except for Virginia Slims Gold) were purchased from local retailers, chosen for their popularity in the local area (Dauphin and Lebanon Counties, PA) based on self-report from the retailers and their combined share of the US cigarette market (these brands represent approximately 70% of the US market) (Sharma et al., 2015). The 3R4F research cigarette was obtained from the University of Kentucky (Lexington, Kentucky, USA) and was used as a reference cigarette. The cigarettes were stored at −20°C in airtight plastic bags.
2.2 Materials
Acetonitrile (ACN) and concentrated hydrochloric acid (12N HCl) were purchased from Fisher Scientific (Pittsburgh, PA, USA) and used as supplied. Diglyme and dinitrophenylhydrazones of formaldehyde, acetaldehyde, crotonaldehyde, propionaldehyde, and MEK were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as supplied. 2,4-Dinitrophenylhydrazine (DNPH) was purchased from BOC Sciences (Shirley, NY, USA) and recrystallized before use to remove water, which can affect the reactivity of DNPH with carbonyls (Risner and Martin, 1994). Recrystallization was done by dissolving 17 g DNPH in 350 mL acetonitrile, heating this solution at 70°C for 60 min., and then cooling to room temperature. The crystals were collected via vacuum filtration, and then stored in a desiccator.
2.3 Mainstream Smoke Generation
The cigarettes were conditioned for testing by removing them from cold storage and placing them in a constant humidity chamber (60% relative humidity, 22±1°C), for at least 48 h before smoking. Mainstream smoke was generated by a 30-port smoking machine (Jaeger-Baumgartner, CSM JB2080). Five cigarettes were smoked simultaneously on the machine under a FTC smoking protocol: 35 mL puff volume, 2 s puff duration, and 60 s puff interval. We tested for breakthrough, which is when any carbonyl passes through the capturing solution and thus is not captured, by adding a second impinger in line. Breakthrough was minimal (~2%) for all carbonyls when testing between one and five cigarettes on the smoking machine, but did become an issue when testing ten or more cigarettes. Thus, we limited our study to five or less cigarettes per collection.
2.4 Derivatization of Carbonyls
DNPH solution was made as described previously (Risner and Martin, 1994) by dissolving 1.0 g recrystallized DNPH in a mixture of 50 mL diglyme, 360 μL 12N HCl, and 150 mL ACN Mainstream smoke generated from five cigarettes of each brand as described above was passed directly into an impinger containing 10 mL of DNPH solution placed after the pump. The sample was then transferred into a 20 mL scintillation vial and stored at 4°C until HPLC-UV analysis, which was performed within 5 days of collection. We performed a minimum of two replicates of each collection (n = 2–4 for all experiments). Day to day assay variation was low (Coefficient of variation (CV) = 5%) for total carbonyls in 3R4F cigarettes (Table 1). When the impinger was moved upstream of the pump, yields were increased for acetaldehyde only (15%; p-value: 0.04); however, it was difficult to maintain a consistent flow rate in this configuration, so it was not used for further experiments.
Table 1.
Carbonyl delivery by cigarette branda
| Brands | Flavorb | Form-aldehyde (μg/cigarette) | Acetaldehyde (μg/cigarette) | Propion-aldehyde (μg/cigarette) | Croton-aldehyde (μg/cigarette) | Methyl Ethyl Ketone (μg/cigarette) | Total Carbonyls (μg/cigarette) |
|---|---|---|---|---|---|---|---|
| 3R4F | R | 19 ± 5 | 489 ± 20 | 46 ± 5 | 19 ± 3 | 72 ± 7 | 644 ± 26 |
| American Spirit Mellow Menthol | M | 52 ± 32 | 345 ± 22 | 33 ± 2 | 10 ± 1 | 50 ± 6 | 490 ± 54 |
| American Spirit Menthol | M | 39 ± 22 | 397 ± 56 | 38 ± 5 | 16 ± 1 | 61 ± 7 | 550 ± 65 |
| American Spirit Orange | R | 69 ± 42 | 173 ± 29 | 15 ± 7 | 6 ± 1 | 28 ± 1 | 292 ± 90 |
| American Spirit Turquoise | R | 42 ± 18 | 270 ± 19 | 27 ± 3 | 11 ± 4 | 51 ± 6 | 401 ± 10 |
| Camel Blue | R | 45 ± 4 | 435 ± 82 | 43 ± 8 | 13 ± 4 | 58 ± 16 | 593 ± 100 |
| Camel Filters | R | 38 ± 33 | 391 ± 2 | 36 ± 1 | 14 ± 4 | 57 ± 3 | 536 ± 30 |
| Camel Silver | R | 23 ± 28 | 201 ± 24 | 21 ± 3 | 7 ± 1 | 34 ± 4 | 286 ± 57 |
| Eagle20’s Gold Menthol | M | 45 ± 17 | 611 ± 36 | 49 ± 7 | 24 ± 5 | 76 ± 18 | 804 ± 42 |
| Kool | M | 35 ± 15 | 471 ± 61 | 46 ± 6 | 18 ± 10 | 69 ± 13 | 639 ± 70 |
| L&M Blue | R | 29 ± 4 | 546 ± 2 | 52 ± 3 | 23 ± 1 | 78 ± 2 | 728 ± 10 |
| L&M Menthol | M | 37 ± 9 | 489 ± 34 | 47 ± 1 | 22 ± 2 | 75 ± 10 | 670 ± 50 |
| L&M Red | R | 43 ± 24 | 377 ± 17 | 37 ± 1 | 13 ± 1 | 51 ± 2 | 520 ± 26 |
| Marlboro Gold | R | 19 ± 10 | 352 ± 27 | 34 ± 3 | 13 ± 1 | 54 ± 1 | 472 ± 39 |
| Marlboro Menthol | M | 44 ± 12 | 494 ± 50 | 48 ± 4 | 20 ± 1 | 72 ± 6 | 677 ± 67 |
| Marlboro Red | R | 53 ± 18 | 407 ± 80 | 36 ± 5 | 36 ± 8 | 66 ± 10 | 597 ± 100 |
| Marlboro Silver | R | 33 ± 43 | 251 ± 6 | 23 ± 1 | 14 ± 9 | 41 ± 2 | 362 ± 39 |
| Newport | M | 31 ± 17 | 521 ± 43 | 50 ± 12 | 33 ± 13 | 75 ± 5 | 710 ± 66 |
| Newport Red | R | 33 ± 24 | 483 ± 26 | 44 ± 3 | 22 ± 14 | 69 ± 5 | 651 ± 29 |
| Pall Mall Menthol | M | 43 ± 17 | 460 ± 26 | 41 ± 6 | 17 ± 1 | 73 ± 5 | 633 ± 40 |
| Pall Mall Orange | R | 22 ± 34 | 194 ± 42 | 19 ± 5 | 7 ± 1 | 34 ± 4 | 275 ± 84 |
| Pall Mall Red | R | 21 ± 4 | 323 ± 47 | 28 ± 2 | 30 ± 1 | 55 ± 5 | 457 ± 52 |
| Parliament White | R | 12 ± 7 | 312 ± 33 | 30 ± 4 | 16 ± 8 | 53 ± 5 | 423 ± 44 |
| Parliament White Menthol | M | 35 ± 3 | 404 ± 22 | 37 ± 3 | 17 ± 2 | 55 ± 3 | 548 ± 31 |
| Pyramid Gold Menthol | M | 36 ± 4 | 485 ± 10 | 45 ± 2 | 16 ± 4 | 64 ± 1 | 647 ± 18 |
| Pyramid Red | R | 54 ± 26 | 448 ± 32 | 42 ± 3 | 17 ± 4 | 57 ± 9 | 617 ± 30 |
| Salem | M | 52 ± 1 | 568 ± 84 | 47 ± 14 | 19 ± 2 | 78 ± 6 | 764 ± 101 |
| Virginia Slims Gold | R | 40 ± 22 | 260 ± 37 | 22 ± 3 | 10 ± 4 | 41 ± 4 | 373 ± 57 |
Note: All cigarettes were from king-sizes hard packs (boxes), except 3R4F (soft pack) and Virginia Slims Gold (100s).
Values are mean ± SD (n=2–4)
R = Regular; M = Menthol
2.5 HPLC-UV Analysis
High performance liquid chromatography with ultraviolet detection (HPLC-UV) analyses were performed using a binary system consisting of two Waters (Milford, MA, USA) 510 pumps, a Waters 440 UV absorbance detector, and a Hitachi (Tokyo, Japan) D-2500 Integrator. Compounds were separated using a Bondclone C18 column (10 μm × 300 mm × 3.9 mm; Phenomenex, Torrance, CA, USA) using two mobile phases: water (A) and acetonitrile (B). The elution gradient parameters are: 0 min., 90% A, 10% B; 20 min., 10% A, 90% B; 25 min., 10% A, 90% B, 27 min., 90% A, 10% B; and 37 min. 90% A, 10 % B. The flow rate is 1.0 mL/min. for all time steps, and the detection wavelength was 254 nm. All sample injections were 10 μL and were manually injected. All measurements were carried out at room temperature (22±1°C). Supplementary Figure 1 is a representative chromatogram. This method was found to have good precision (CV: 6–12%) for all carbonyls tested, which was determined by 12 replicate injections of a collected cigarette sample. Accuracy was determined through spiking a sample with known amounts of carbonyl standards and found to have a CV of 4%.
2.6 GC-MS Analysis
All peaks, except crotonaldehyde, were verified by collecting the eluting peaks from the HPLC-UV and analyzing by GC-MS. To do this, an Agilent Technologies 7890A gas chromatograph (Agilent Technolgies, Inc., Santa Clara, CA) fitted with a Gerstel MPS2 autosampler (Gerstel GMbH & Co. KG. Mülheim an der Ruhr, Germany) was used in combination with an Agilent 5975C mass selective detector. The instrument and autosampler were controlled by Agilent MassHunter GCMS Acquisition software (Version B.07.00 SP2.1654) and Gerstel Maestro 1 software (Version1.2.20.41), respectively. The GC was fitted with a J&W VF-35ms capillary column with dimensions 60 m × 0.25 mm × 0.25 μm. The inlet was fitted with a borosilicate glass single taper inlet liner with wool (6.5 mm o.d., 4.0 mm i.d., 78.5 mm long) (Restek, Bellefonte, PA). Helium (ultra-high purity) was used as the carrier gas at a constant flow rate of 1.4 mL/min. Depending upon analyte concentration within the sample, between one and three microliters of sample were introduced to an injector operated in split-less mode and maintained at a temperature of 270°C. The splitter was opened to 71:1 after 60 s. The GC oven was first held at 50°C for 2.5 minutes, then increased to 200°C at a rate of 15°C/min, and finally increased to 300°C at a rate of 5°C/min. The MS transfer line was held at 280°C. The mass spectrometer was operated in full scan mode across an ion range of m/z 35 to 350. Data analysis was performed using the Agilent MSD Enhanced ChemStation software package (Version F.01.00.1903).
2.7 Statistics
Summary statistics and boxplots are presented for the major variables of carbonyls. The associations between carbonyls and free radicals are estimated and tested using regression analyses using SAS software Version 9.4 of the SAS System for Windows x64 Systems (SAS Institute Inc., Cary, NC, USA). Linear regression was used to determine the significance of ventilation and flavor group effects and performed using R 3.3. (R Core Team, 2016) Ventilation and TPM values were those found by Goel et al. (Goel et al., 2017).
3. Results
3.1 Cigarettes
The brand information is given in Supplementary Table 1. All twenty-seven cigarette brands, the research cigarette, and their corresponding carbonyl delivery per cigarette and their respective flavors are listed in Table 1. All cigarettes were 80–85 mm, except for Virginia Slims (100 mm). Flavor refers to if the cigarettes were labelled as menthol.
3.2 Carbonyl Levels and Variation
Carbonyl levels were determined by HPLC-UV, and peak-compound assignments were matched to standards by retention times and later verified by GC-MS. Precision of peak areas were found to be between 6 – 12% for all compounds. Acrolein co-eluted with acetone on the HPLC-UV, thus individual levels could not be determined. Based on the five that were readily quantified, acetaldehyde was the most abundant and least variable, accounting for, on average, 72 ± 3% (CV =5%) of total carbonyls across all brands. This was followed by MEK at 11 ± 1% (CV = 8%) and propionaldehyde at 7 ± 1% (CV = 7%). Crotonaldehyde was the least abundant, but second most variable at 3±1% (CV = 33%). The most variation was observed with formaldehyde levels, which were approximately 7 ± 4% (CV = 54%) of total carbonyls.
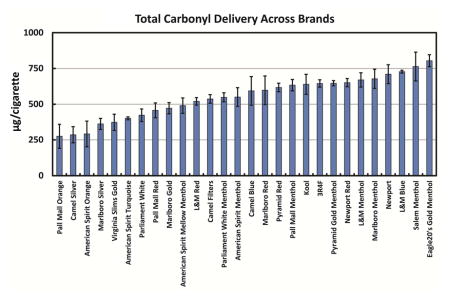
There is a large variation in carbonyl production across brands (Table 1). For individual carbonyls, levels ranged between 3- and 6-fold between brands with the greatest variation observed for formaldehyde (6-fold) and crotonaldehyde (6-fold) and least variation observed for MEK (3-fold) and propionaldehyde (3-fold). Total carbonyl levels ranged three-fold (275 ± 84 μg/cigarette for Pall Mall Orange to 804 ± 42 μg/cigarette for Eagle20’s Gold Menthol).
3.3 Correlations between Carbonyls
To examine for potential associations between carbonyls, coefficients of determination (r2) were calculated (Table 2). Acetaldehyde, propionaldehyde, and MEK were highly correlated with one another (r2: 0.90–0.95, P<0.001). Correlations, albeit weaker, were also observed between each of these carbonyls and crotonaldehyde (r2: 0.36–0.51, P<0.01). Formaldehyde was the sole carbonyl that was not correlated with the rest, which could be predicted by the high variation we observed for formaldehyde.
Table 2.
Correlations between carbonyl and free radical deliveries across different brands of cigarettes.
| Formaldehyde | Acetaldehyde | Propionaldehyde | Crotonaldehyde | Methyl Ethyl Ketone (MEK) | Total Carbonyls | Ventilation | |
|---|---|---|---|---|---|---|---|
| Formaldehyde | |||||||
| Acetaldehyde | 0.009 (0.64) | ||||||
| Propionaldehyde | 0.001 (0.87) | 0.953 (< 0.001) | |||||
| Crotonaldehyde | (−) 0.005 (0.72) | 0.395 (0.003) | 0.356 (0.008) | ||||
| MEK | 0.000 (0.97) | 0.909 (< 0.001) | 0.895 (< 0.001) | 0.510 (< 0.001) | |||
| Free Radicals (Gas Phase) | (−) 0.107 (0.09) | 0.416 (0.002) | 0.418 (0.002) | 0.209 (0.01) | 0.327 (0.002) | 0.370 (0.006) | (−) 0.336 (0.001) |
| Free Radicals (Particulate Phase) | 0.012 (0.50) | 0.311 (0.002) | 0.236 (0.009) | 0.054 (0.23) | 0.172 (0.03) | 0.268 (0.005) | (−) 0.313 (0.002) |
| TPM | 0.0003 (0.93) | 0.440 (< 0.001) | 0.421 (0.002) | 0.225 (0.01) | 0.401 (0.003) | 0.435 (< 0.001) | (−) 0.564 (< 0.001) |
| Ventilation | 0.009 (0.63) | (−) 0.589 (< 0.001) | (−) 0.589 (< 0.001) | (−) 0.411 (0.002) | (−) 0.526 (< 0.001) | (−) 0.600 (< 0.001) |
Values are r2 with negative sign added to indicate a negative correlation and corresponding p-values in parentheses.
Interestingly, the brand with the highest delivery differed for each individual carbonyl (formaldehyde [Camel Silver], acetaldehyde [Eagle20’s Gold Menthol], propionaldehyde [L&M Blue], crotonaldehyde [Marlboro Red], and MEK [Salem]) while all but one of the lowest values were from the same brand (Camel Silver). However, Camel Silver is not the lowest in total carbonyls despite being lowest in four of the five individual carbonyls as it produced the highest value for formaldehyde, stressing the importance of monitoring all carbonyls individually.
3.4 Ventilation, TPM, and Menthol
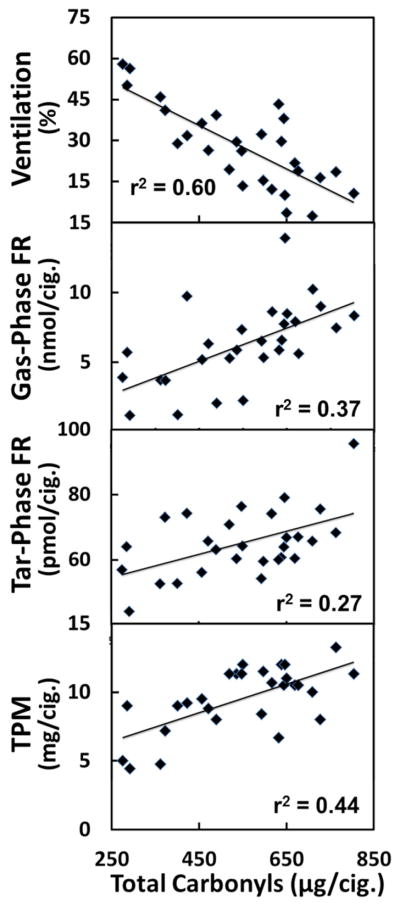
We conducted regressions to determine if levels of carbonyls were associated with ventilation and TPM (using ventilation and TPM data from Goel et al., 2017). We did find significant correlations between carbonyls and TPM, with the exception of formaldehyde (r2: 0.22–0.44; P ≤ 0.01; Table 2; Figure 2). We find that ventilation has a significant negative relationship with all carbonyl deliveries, except for formaldehyde (r2: 0.41–0.59; P ≤ 0.002; Table 2). In addition, TPM and ventilation also have a significant negative correlation with each other (r2: 0.56; P < 0.001).
Figure 2.
Correlations between cigarette ventilation, gas-phase free radicals, particulate-phase free radicals, and TPM with total carbonyls. All correlations are statistically significant (P ≤ 0.05).
We find, on a per cigarette basis, menthol cigarettes tended to produce significantly higher levels for all carbonyls than non-menthol brands (Figure 1). Menthol brands themselves only varied 2-fold for each carbonyl and total carbonyls compared to 3- to 6-fold among non-menthol brands and all brands together. After adjustment for ventilation, acetaldehyde, propionaldehyde, MEK, and total carbonyls remain significant (P ≤ 0.05), suggesting that while our menthol brands are higher from containing less ventilation overall, it is not the sole contribution affecting the differences in carbonyl delivery.
Figure 1.
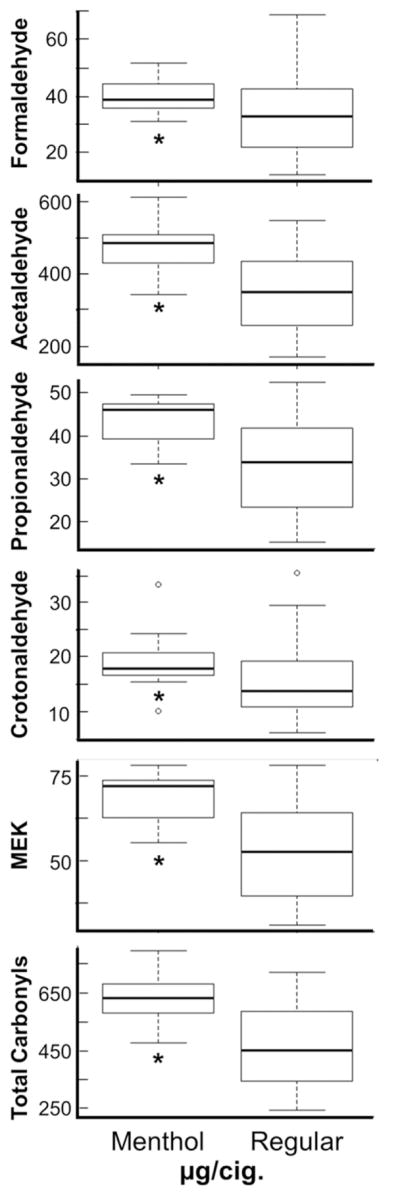
Boxplot results comparing flavor groups on a per cigarette basis. All units are μg/cigarette. After adjustment for ventilation, acetaldehyde, propionaldehyde, MEK, and total carbonyls remain significant. *P ≤ 0.05
3.5 Correlation with Free Radicals
We performed a correlational analysis with each carbonyl along with total carbonyls and both gas-phase and particulate-phase free radicals (Table 2; (Goel et al., 2017). Significant rank correlations were observed between all carbonyls, except formaldehyde, and gas-phase free radicals. For particulate-phase free radicals significant correlations were only observed with acetaldehyde and propionaldehyde. When total carbonyls were considered, significant correlations were observed for both gas-phase (r2: 0.37; P: 0.006) and particulate-phase (r2: 0.27; P: 0.005) free radicals (Figure 2). After adjusting for ventilation, which was found to be significantly correlated with free radicals and carbonyls, we find that acetaldehyde and propionaldehyde remain significantly but weakly correlated with gas-phase free radicals (r2: 0.06; P ≤ 0.048). In addition, no carbonyls were significantly correlated with tar-phase radicals, suggesting most of the effects were driven by differences in ventilation (Table 3).
Table 3.
Correlations between carbonyl and free radical deliveries across different brands of cigarettes adjusted for ventilation.
| Formaldehyde | Acetaldehyde | Propionaldehyde | Crotonaldehyde | Methyl Ethyl Ketone (MEK) | Total Carbonyls | |
|---|---|---|---|---|---|---|
| Free Radicals (Gas Phase) | 0.120 (0.06) | 0.060 (0.048) | 0.062 (0.046) | 0.010 (0.50) | 0.035 (0.17) | 0.039 (0.12) |
| Free Radicals (Particulate Phase) | 0.050 (0.26) | 0.050 (0.22) | 0.005 (0.60) | 0.023 (0.32) | 0.0001 (0.94) | 0.010 (0.42) |
Values are partial r2 value with corresponding p-values in parentheses.
4. Discussion
Here we observe the levels of five major carbonyls in mainstream smoke from 27 popular brands of cigarettes. We observe a wide variation in levels of carbonyls examined. The overall variation in levels across brands remains quite large even after adjusting for ventilation, which accounts for 40 to 60% of the variation depending on the carbonyl. From comparing these results with those for free radicals (Goel et al., 2017), we find that carbonyl levels are significantly correlated with the levels of free radicals, the other major class of oxidants found in cigarette smoke, across cigarette brands.
Among all the carbonyls measured, acetaldehyde was the most abundant and varied 4-fold across brands, similar to differences previously observed between other US cigarette brands and types (Counts et al., 2005; Pazo et al., 2016; Roemer et al., 2003). Crotonaldehyde, propionaldehyde, and MEK also had significant variation across brands (3–6-fold), also similar to previous reports (Counts et al., 2005; Pazo et al., 2016; Roemer et al., 2003). Carbonyl levels were highly correlated with one another (except formaldehyde), suggesting that their production during pyrolysis may involve similar factors which may differ across brands based on differences in cigarette design and/or tobacco blends. Thus, smokers who smoke certain products may be exposed to higher levels of carbonyls and, thus, may have a greater negative health impact especially when these doses are extrapolated over a lifetime of smoking. Acrolein, another important carbonyl which is thought to play an important role in several smoking-induced illnesses, was not measured due to its co-elution with acetone on the HPLC., However, acrolein levels in tobacco smoke have previously been shown to be highly correlated with the other major carbonyls (except formaldehyde) across tobacco blends (Ding et al., 2015).
In comparison to the other carbonyls, formaldehyde also varied greatly in levels across brands (6-fold), but they did not correlate with the levels of other carbonyls. The range of formaldehyde levels across brands was similar to that previously observed (Counts et al., 2005; Roemer et al., 2003). While it is not known why formaldehyde differs from the other carbonyls, it is possible much of the formaldehyde in tobacco smoke may result from formaldehyde present in the tobacco leaf itself and not simply as a product of combustion as are the other carbonyls. To further support this, Ding et al. (Ding et al., 2015) reported that formaldehyde in mainstream smoke differs greatly (4-fold) between bright and burley tobacco, which are the main two blends in U.S. cigarettes (Hoffmann et al., 2001). Thus, altering the amounts of bright and burley slightly between cigarettes could cause great differences in formaldehyde produced. In addition, formaldehyde is near equally split between the particulate phase (39%) and gas phase (61%) of cigarette smoke while the other four are almost entirely in the gas phase (85–99%) (Pang and Lewis, 2011). Overall, these findings demonstrate the need to consider formaldehyde as distinctly different from other carbonyls in terms of production in cigarette smoke.
In our studies, the lowest levels for almost all carbonyls were consistently observed for American Spirit sub-brands. While this is consistent with the free radical findings, it does contrast with a previous study (Pazo et al., 2016) that found an American Spirit sub-brand to be one of their highest. However, the single sub-brand used in that study was not in our study (American Spirit Blue; personal correspondence), thus the difference might arise from that particular sub-brand. When we compare our results to those observed by Pazo et al. (Pazo et al., 2016) for the sub-brands that are the same, our levels are similar for most carbonyls; however, the levels of acetaldehyde are less than they observed (e.g., 3R4F: 610±170 vs. 489±20 ug acetaldehyde/cig.). The difference likely arises from the different methods used for analysis (gas bag sampling method vs. impinger-derivatization method) as the values reported by CORESTA, which also uses an impinger-derivatization method, also report lower values, more similar to what we report (3R4F: 538±28 ug acetaldehyde/cig.) (CORESTA, 2014).
As with the individual carbonyls, total carbonyls varied greatly across brands (3-fold; Table 1). While the nature of this variation is unknown, it could result from a variety of design differences across brands including tobacco blend, ventilation, and flavor additives. Tobacco blend is intrinsically tied to carbonyl yields, has been previously shown with different types of tobacco yielding dissimilar amounts of carbonyls (Ding et al., 2015). Filter ventilation differs amongst cigarettes and allows for differences in combustion temperature and dilution of the delivered smoke (Kozlowski et al., 1998; O’Connor et al., 2008). We found a negative correlation between ventilation and carbonyl delivery (r2: 0.41–0.60), consistent with previous findings where carbonyl delivery was increased by blocking ventilation (Pazo et al., 2016).
We found higher levels for all carbonyls in menthol compared to non-menthol brands (Figure 1). A previous paper reported a trend toward higher carbonyl values for menthol cigarettes; however, these differences were not significant (Bodnar et al., 2012). Menthol cigarettes differ from non-menthol brands in numerous ways including a higher menthol content (Celebucki et al., 2005) and lower level of ventilation (Pazo et al., 2016). While the majority of menthol brands tested were not highly ventilated, most carbonyls remained significantly correlated after adjusting for ventilation. Thus, it remains possible that in addition to ventilation, menthol flavoring itself may be playing a role. Indeed, the potential impact of menthol as a risk factor for tobacco-related illnesses remains under debate (Heck, 2010; Hoffman, 2011; Hoffman and Simmons, 2011; Salgado and Glantz, 2011; Werley et al., 2007).
Results also showed that TPM was correlated with most carbonyl levels, helping to explain ~50% of the variation observed between brands (Table 2). TPM, which consists of tar and water formed during combustion, contains many harmful compounds as well, such as polyaromatic hydrocarbons and tobacco-specific nitrosamines. The one exception was formaldehyde, which was not correlated with TPM, further suggesting its levels are affected differently than the other carbonyls. Since levels of TPM are generally impacted by combustion, this association supports shared mechanisms for the formation of TPM constituents and carbonyls tied to the pyrolysis of the plant material. TPM can also be influenced by ventilation as noted by the strong correlation we observe between the two, suggesting that the differences in TPM should be, at least partially, corrected for by correcting for ventilation.
Our study establishes, for the first time, that total carbonyls are significantly correlated with free radicals by comparing our carbonyl levels to Goel et al.’s data on free radicals in the same 27 brands smoked under identical experimental conditions. This is important as these are the two major classes of oxidants produced in cigarette smoke from the process of pyrolysis and incomplete combustion while also being considered the source of most oxidative damage in smokers. By understanding how these levels relate across brands, we can gain insight into the potential total oxidant harm to which a smoker is exposed from the pyrolysis and incomplete combustion of tobacco.
The gas-phase radicals correlated with each carbonyl, except formaldehyde, perhaps reflecting a common mechanism of formation involving combustion. We further examined this correlation through adjusting for ventilation, finding that only weak, if any, correlations remain (Table 3); this strongly suggests that ventilation is the main common factor that these two oxidants are affected by. Ventilation, however, does not explain the wide variation in the brands in terms of oxidants as it accounts for ~55% of the variation for carbonyls and only 30% of the variation in free radicals levels. Knowing the relationship of these oxidant classes and the main cigarette design feature that affects it will help us better estimate the relative harm of these products.
On the other hand, the particulate-phase radicals show a moderate correlation with carbonyls, observing significant correlations with three of the five carbonyls: acetaldehyde, propionaldehyde, and MEK (Table 2), and these correlations disappear when adjusting for ventilation (Table 3). This lack of relationship for some carbonyls could be a result of the low overall variation in particulate-phase radicals compared to gas phase radicals, changing only 2-fold over all the brands compared to the 12-fold variation in gas phase radicals. Using the present results, a better prediction of the total oxidant exposure from pyrolysis to a smoker of different brands can be made. The strong correlation between these different classes of oxidants suggests that there is wide brand-to-brand variation in overall oxidant delivery which may be associated with potential difference in harm.
Overall, this study helps underline the importance of understanding and acknowledging the variation in delivery of oxidants between cigarette brands. We display clearly that carbonyls and free radicals do share a positively correlated relationship, suggesting that reductions in total oxidant delivery may be possible. This is important as it has been proposed previously (Burns et al., 2007) that limitations be placed on the amounts of toxicants a cigarette can produce, which the FDA can impose as a part of a total harm reduction strategy. Here we provide new information on the relative oxidant levels produced by commercially available cigarettes, which can help guide potential future regulatory strategies. More information is needed as some brands might be low in oxidants, yet be high in other dangerous toxicants. We also do not include other oxidants produced from combustion, such as polyaromatic hydrocarbons, or that are present in the tobacco itself, such as metals. Finally, we acknowledge puff topography factors, such as puff volume and duration, can have a direct impact on the delivery of smoke constituents. While the ISO protocol used in this study is more representative of light smokers, topography profiles for a majority of smokers fall along a continuum between ISO and more intense protocols such as the Health Canada Intense (HCI) method. Thus, differences in carbonyl and free radical delivery under conditions of different puffing topography will need to be examined in future investigations. We do not fully understand all the factors contributing to brand variation in oxidant production, and so further research is required to guide potential future regulatory recommendations. However, the magnitude of the brand variations found here suggests that such research may eventually help identify ways to reduce the oxidant delivery of cigarettes and so reduce smoking-caused disease.
Supplementary Material
Highlights.
Carbonyls and free radicals are two of the major classes of oxidants in tobacco smoke.
Carbonyls varied widely (3-6-fold) across cigarette brands with ventilation accounting for only half of this variation.
Carbonyls are correlated with gas- and particulate-phase free radicals.
However, these correlations are eliminated when adjusting for ventilation.
Acknowledgments
We would like to thank Laura Homich for her assistance in confirming our compounds by GC-MS.
Funding Sources
This work was supported in part by the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration (under Award Number P50-DA-036107). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
ABBREVIATIONS
- TPM
total particulate matter
- CV
coefficient of variation
- ρ
Spearman’s rank correlational coefficient
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Supporting Information. Brand details and a representative chromatogram are available in the Supporting Information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker RR, Pereira da Silva JR, Smith G. The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives. Food Chem Toxicol. 2004a;42(Suppl):S3–37. doi: 10.1016/S0278-6915(03)00189-3. [DOI] [PubMed] [Google Scholar]
- Baker RR, Pereira da Silva JR, Smith G. The effect of tobacco ingredients on smoke chemistry. Part II: Casing ingredients. Food Chem Toxicol. 2004b;42(Suppl):S39–52. doi: 10.1016/j.fct.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Bodnar JA, Morgan WT, Murphy PA, Ogden MW. Mainstream smoke chemistry analysis of samples from the 2009 US cigarette market. Regulatory toxicology and pharmacology: RTP. 2012;64:35–42. doi: 10.1016/j.yrtph.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Burney P, Jithoo A, Kato B, Janson C, Mannino D, Niżankowska-Mogilnicka E, Studnicka M, Tan W, Bateman E, Koçabas A, Vollmer WM, Gislason T, Marks G, Koul PA, Harrabi I, Gnatiuc L, Buist S. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty—a BOLD analysis. Thorax. 2014;69:465–473. doi: 10.1136/thoraxjnl-2013-204460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DM, Dybing E, Gray N, Hecht S, Anderson C, Sanner T, O’Connor R, Djordjevic M, Dresler C, Hainaut P, Jarvis M, Opperhuizen A, Straif K. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob Control. 2007;17:132–141. doi: 10.1136/tc.2007.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebucki CC, Wayne GF, Connolly GN, Pankow JF, Chang EI. Characterization of measured menthol in 48 U.S. cigarette sub-brands. Nicotine Tob Res. 2005;7:523–531. doi: 10.1080/14622200500186270. [DOI] [PubMed] [Google Scholar]
- Chen MS, Xu ZQ, Chen G, Wang H, Yin CY, Zhou ZL, Sun WF, Li Y, Zhong F. The influence of exogenous fiber on the generation of carbonyl compounds in reconstituted tobacco sheet. J Anal Appl Pyrol. 2014;105:227–233. [Google Scholar]
- Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environmental health perspectives. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORESTA; CORESTA, editor. Recommended Method N° 74: Determination of Selected Carbonyls in Mainstream Cigarette Smoke by HPLC. CORESTA; Paris, France: 2014. [Google Scholar]
- Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Regulatory toxicology and pharmacology: RTP. 2004;39:111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regulatory toxicology and pharmacology: RTP. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:365. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Xizheng Y, Wong J, Chan M, Watson CH. In Situ Derivatization and Quantification of Seven Carbonyls in Cigarette Mainstream Smoke. Chem Res Toxicol. 2015;29:125–131. doi: 10.1021/acs.chemrestox.5b00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich DJ, Fieblekorn RT, Bevan MJ, Rushforth D, Murphy JJ, Ashley M, McAdam KG, Liu C, Proctor CJ. Approaches for the design of reduced toxicant emission cigarettes. SpringerPlus. 2014;3:1–23. doi: 10.1186/2193-1801-3-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Hill AB. Smoking and carcinoma of the lung. Br Med J. 1950;2:739. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R, Bitzer Z, Reilly SM, Trushin N, Foulds J, Muscat J, Liao J, Elias RJ, Richie JP., Jr Variation in Free Radical Yields from U.S. Marketed Cigarettes. Chem Res Toxicol. 2017;30:1038–1045. doi: 10.1021/acs.chemrestox.6b00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell BB, Gutteridge JMC. Free Radicals in Biology and Medicine. 5. Oxford University Press; United Kingdom: 2015. [Google Scholar]
- Hammond D, O’Conner RJ. Constituents in tobacco and smoke emissions from Canadian cigarettes. Tob Control. 2008;17:i24–i31. doi: 10.1136/tc.2008.024778. [DOI] [PubMed] [Google Scholar]
- Heck JD. A review and assessment of menthol employed as a cigarette flavoring ingredient. Food Chem Toxicol. 2010;48(Suppl 2):S1–38. doi: 10.1016/j.fct.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Hoffman AC. The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tobacco induced diseases. 2011;9(Suppl 1):S7. doi: 10.1186/1617-9625-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Simmons D. Menthol cigarette smoking and nicotine dependence. Tobacco induced diseases. 2011;9(Suppl 1):S5. doi: 10.1186/1617-9625-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Djordjevic MV, Brunnemann KD. Changes in Cigarette Design and Composition Over Time and How They Influence the Yields of Smoke Constituents. J Smoking Relat Dis. 1995;6:9–23. [Google Scholar]
- Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: A controversial issue. A tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Mehta NY, Sweeney CT, Schwartz SS, Vogler GP, Jarvis MJ, West RJ. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob Control. 1998;7:369–375. doi: 10.1136/tc.7.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupski WC. The peripheral vascular consequences of smoking. Ann Vasc Surg. 1991;5:291–304. doi: 10.1007/BF02329389. [DOI] [PubMed] [Google Scholar]
- Kundu TK, Hille R, Velayutham M, Zweier JL. Characterization of superoxide production from aldehyde oxidase: an important source of oxidants in biological tissues. Archives of biochemistry and biophysics. 2007;460:113–121. doi: 10.1016/j.abb.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcilla A, Martinez I, Berenguer D, Gomez-Siurana A, Beltran MI. Comparative study of the main characteristics and composition of the mainstream smoke of ten cigarette brands sold in Spain. Food Chem Toxicol. 2012;50:1317–1333. doi: 10.1016/j.fct.2012.01.046. [DOI] [PubMed] [Google Scholar]
- O’Connor RJ, Hammond D, McNeill A, King B, Kozlowski LT, Giovino GA, Cummings KM. How do different cigarette design features influence the standard tar yields of popular cigarette brands sold in different countries? Tob Control. 2008;17:I1–I5. doi: 10.1136/tc.2006.019166. [DOI] [PubMed] [Google Scholar]
- Pang X, Lewis AC. Carbonyl compounds in gas and particle phases of mainstream cigarette smoke. Sci Total Environ. 2011;409:5000–5009. doi: 10.1016/j.scitotenv.2011.07.065. [DOI] [PubMed] [Google Scholar]
- Pazo DY, Moliere F, Sampson MM, Reese CM, Agnew-Heard KA, Walters MJ, Holman MR, Blount BC, Watson C, Chambers DM. Mainstream Smoke Levels of Volatile Organic Compounds in 50 US Domestic Cigarette Brands Smoked with the ISO and Canadian Intense Protocols. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environmental health perspectives. 1997;105(Suppl 4):875–882. doi: 10.1289/ehp.97105s4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- Risner CH, Martin P. Quantitation of formaldehyde, acetaldehyde, and acetone in sidestream cigarette smoke by high-performance liquid chromatography. J Chromatogr Sci. 1994;32:76–82. doi: 10.1093/chromsci/32.3.76. [DOI] [PubMed] [Google Scholar]
- Roemer E, Schorp MK, Piadé JJ, Seeman JI, Leyden DE, Haussmann HJ. Scientific assessment of the use of sugars as cigarette tobacco ingredients: A review of published and other publicly available studies. Crit Rev Toxicol. 2012;42:244–278. doi: 10.3109/10408444.2011.650789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, Podraza KF. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2003;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Salgado MV, Glantz SA. Direct disease-inducing effects of menthol through the eyes of tobacco companies. Tob Control. 2011;20(Suppl 2):ii44–48. doi: 10.1136/tc.2010.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman JI, Laffoon SW, Kassman AJ. Evaluation of Relationships Between Mainstream Smoke Acetaldehyde and “Tar” and Carbon Monoxide Yields in Tobacco Smoke and Reducing Sugars in Tobacco Blends of U.S. Commercial Cigarettes. Inhal Toxicol. 2003;15:373–395. doi: 10.1080/08958370304461. [DOI] [PubMed] [Google Scholar]
- Sharma A, Fix BV, Delnevo C, Cummings KM, O’Conner RJ. Trends in market share of leading cigarette brands in the USA: national survey on drug use and health 2002–2013. BMJ Open. 2015;6:e008813. doi: 10.1136/bmjopen-2015-008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2014. [Google Scholar]
- U.S. Food and Drug Administration; F.a.D. Administration, editor. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List. Food and Drug Administration; 2012. [Google Scholar]
- Werley MS, Coggins CR, Lee PN. Possible effects on smokers of cigarette mentholation: a review of the evidence relating to key research questions. Regul Toxicol Pharmacol. 2007;47:189–203. doi: 10.1016/j.yrtph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Wooten JB, Chouchane S, McGrath TE. Chapter 2: Tobacco Smoke Constituents Affecting Oxidative Stress. In: Halliwell BB, Poulsen HE, editors. Cigarette Smoke and Oxidative Stress. Springer-Verlag Berlin Heidelberg; Germany: 2006. [Google Scholar]
- Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma: a study of six hundred and eighty-four proved cases. JAMA. 1950;143:329–336. doi: 10.1001/jama.253.20.2986. [DOI] [PubMed] [Google Scholar]
- Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual Healthcare Spending Attributable to Cigarette Smoking: An Update. Am J Prev Med. 2015;48:326–333. doi: 10.1016/j.amepre.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher C, Halbert R, Dubois R, George D, Nonikov D. Smoking-related diseases: the importance of COPD. Int J Tuberc Lung Dis. 2004;8:1423–1428. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.