Figure 1. Selected immune therapies under investigation for renal cell carcinoma (RCC).
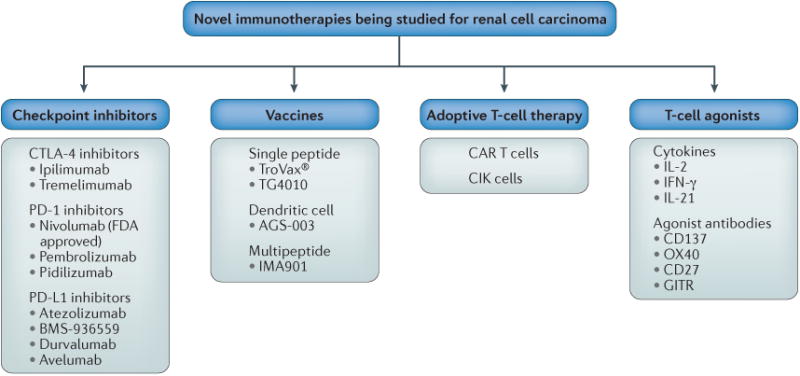
Checkpoint inhibitors under investigation include the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors ipiliimumab and tremelimumab, the programmed cell death protein 1 (PD-1) inhibitors nivolumab (which is FDA approved), pembrolizumab and pidilizumab, and the programmed cell death 1 ligand 1 (PD-L1) inhibitors atezolizumab, BMS-936559, durvalumab, and avelumab. Vaccine strategies investigated in RCC include the single peptide vaccines TroVax® and TG4010, the dendritic cell vaccine AGS-003, and the multipeptide vaccine IMA901. Adoptive T-cell therapies such as chimeric antigen receptor (CAR) T cells and cytokine-induced killer (CIK) cells are also being investigated. Multiple T-cell agonists have been or are being studied, including the cytokines IL-2, IFNγ, and IL-21, as well as agonist antibodies to the co-stimulatory molecules CD137, OX40, CD27 and GITR.
