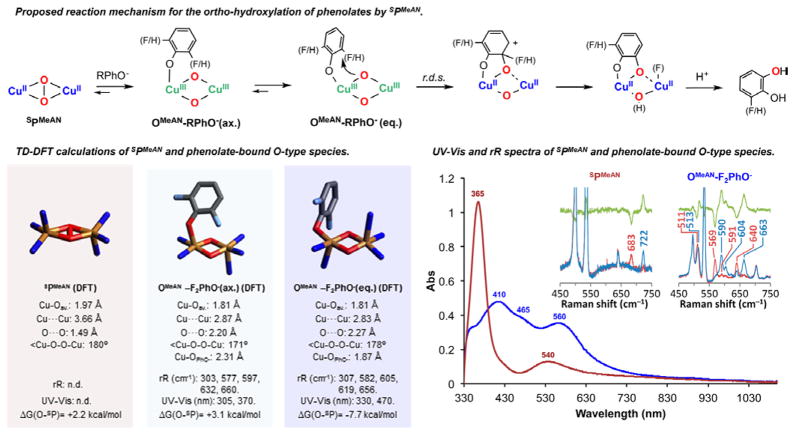
Figure 4.
Top: Postulated reaction mechanism of SPMeAN toward sodium phenolates to generate the corresponding ortho-hydroxylated products through OMeAN-RPhO−. Bottom left: Core structures of DFT-optimized SPMeAN and OMeAN-RPhO− with relevant metrical parameters, calculated Cu–O normal modes, TD-DFT-calculated LMCT energies, and energy of peroxo isomers relative to the corresponding bis(μ-oxo) isomers. Bottom right: Absorption spectra of SPMeAN (brown) and intermediate OMeAN−F2PhO− (blue). Inset: Resonance Raman spectra of SPMeAN (λ = 413.1 nm) and OMeAN−F2PhO− (λ = 568.2 nm) with 16O2 (blue), 18O2 (brown), and the 16O2-18O2 difference spectra (green).