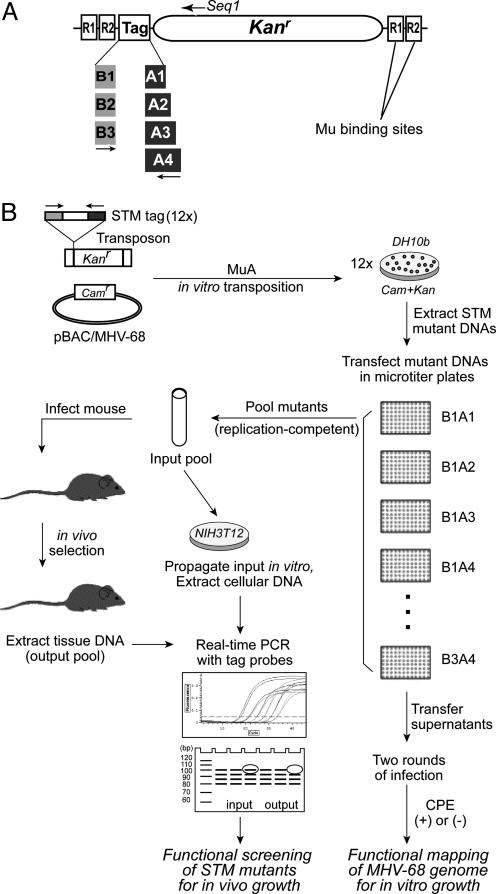
Fig. 1.
STM of MHV-68 for in vivo and in vitro screening. (A) A schematic diagram of STM transposons. Tag sequences are composed of 12 different combinations of three forward and four reverse sequences and a common sequences in the middle, which is designed for real-time PCR. (B) A schematic representation of the MHV-68 STM strategy. By using MuA transposase and an STM transposon with pBAC/MHV-68 as a template, in vitro transposition yields thousands of transposon-inserted mutants. BAC DNAs of STM mutants that have transposon insertion at each ORF of MHV-68 are extracted and transfected into fibroblasts in 96-well plates. After two rounds of infection with transfected supernatants, ORFs are categorized to be essential or nonessential, based on the growth phenotype of a mutant with an insertion proximal to the N terminus of each ORF. Replication-competent mutants are pooled and simultaneously used to infect mice. The inoculum is also propagated in NIH3T12 cells. DNAs from tissues of infected mice (output) and from infected NIH3T12 cells (input) were subjected to real-time PCR, by using STM tag-specific primer sets to determine the fate of each virus within the pool. The copy number of each tag in the input and the output is quantitatively compared to identify the attenuated mutants.