Abstract
Background:
Benign lesion interior to the cavernous sinus (CS) is very rare.
Objective:
In this series we found nonneoplastic lymphatic aggregation and osteoclastoma inside the CS which is very rare and probably not reported in literature. One interesting postoperative complaint of feeling of tickling down of warm water under the skin forehead was found in the patient of inflammatory disease of CS which is not reported in literature. Here we also describe our experiences of microsurgical management of series of benign lesions inside the CS.
Materials and Methods:
Benign mass originated from the content of CS or inner side of walls of CS, confirmed peroperatively were included in this series. Prospectively recorded data of microsurgical management was retrogradely studied.
Results:
Total number of patient was 12. Patient's age range was 30–60 years. Follow-up range was 60 months to 19 months. Three was nonneoplastic lesion (tuberculosis, inflammatory and nonneoplastic lymphoid infiltration). Among the 9 neoplastic lesions, two hemangiomas, two meningiomas, three 6th nerve schwannomas, one osteoclastoma and one epidermoid tumor. Middle cranial fossa-subtemporal extradural approach was used in 9 cases and in two cases extended middle fossa zygomatic approach. New postoperative 3rd nerve palsy developed in 5 cases all recovered completely except one. In seven patients 6th nerve palsy developed after operation; only one recovered. Postoperatively simultaneous 3rd, 4th and 6th nerve palsy developed in four cases. One interesting postoperative complaint of feeling of tickling down of warm water under the skin of left sided forehead was found in the patient of inflammatory disease of CS. Mortality was nil. Total resection was done in 9 cases. There was no recurrence till last follow-up.
Conclusion:
Though decision for microsurgical removal of such lesions is not straight forward. Probably microsurgery is the best option in treating such benign lesions though it may associate with some permanent cranial nerve palsy.
Keywords: Benign cavernous sinus tumor, benign lesion inside the cavernous sinus, cavernous sinus, micro surgical management
Introduction
The cavernous sinus (CS) is a challenging anatomical site even for an expert neurosurgeon. In the past, surgery for CS lesions was associated with a significant risk of complications, so this area was considered a “no man's land” for direct surgical intervention. Inadequate neuroanatomical knowledge and lack of microneurosurgical techniques and skill were the reasons behind this.[1] In 1965 Parkinson[2] first described a direct surgical approach to the CS for a carotid-cavernous fistula (CCF). Since the description of effective skull base approaches and techniques in the 1980s, however, experience has continued to accumulate.[3] Subsequent microanatomical studies and surgical series[1,3,4,5,6,7,8,9,10] have demonstrated that direct approaches to CS lesions can be performed safely and effectively.
In this series, we found nonneoplastic lymphatic aggregation and osteoclastoma inside the CS which are very rare and probably not reported before. One interesting postoperative complaint of feeling of tickling down of warm water under the skin forehead was found in the patient of inflammatory disease of CS which is not reported in literature. Here we also describe our experiences of microsurgical management of intrinsic CS lesions in the aspects of clinical presentation, investigations, microsurgical management and ultimate short-term outcome.
Materials and Methods
A retrospective review of medical records of the patients operated on from January 2007 to December 2012 was conducted after obtaining the Local Ethical Committee approval. Benign mass lesions suspected to be originated from the content of CS or inner side of walls of CS, on the basis of clinical and radiological findings, confirmed peroperatively were included in this series. Tumors that extended into CS from surroundings such as pituitary tumor, trigeminal neurinoma, infratemporal fossa tumor, pharynx and para nasal sinus tumor as well as aneurysm and CCF were excluded. Prospectively recorded data of clinical findings, neuro-imaging data, microsurgical approach and surgical findings, histopathological report and follow-up (clinical and radiological) were retrogradely studied.
Results and Observations
Total number of patients was 12; male 7 and female 5. Left CS lesion was 7 where right sided involvement was 5 only. Patient's age range was 30–60 years; average 41.5 years. Follow-up range was 60 months to 19 months (average - 31.5 months). Details of patients of this series are summarized in Table 1 [Figures 1–4]. Pre- and post-operatively magnetic resonance imaging (MRI) was the main investigation. Preoperatively MRI of brain was done in all cases; computed tomography (CT) scan of brain was done in four cases and digital subtraction angiogram was done one cases only. Common MRI finding was hyper intense contrast enhancing lesion in the CS. All lesions were confined in CS except one where small part of tumor extended into orbit through superior orbital fissure (SOF). Postoperatively contrast MRI was done in 9 cases and only contrast CT scan of brain was done in 3 cases.
Table 1.
Details of all patients
Figure 1.
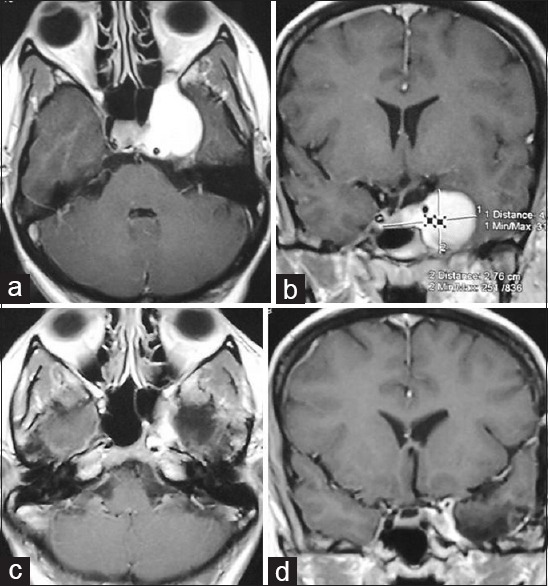
Preoperative contrast magnetic resonance imaging of brain; (a) axial and (b) coronal images showing highly contrast enhancing tumor (haemangioma) in left cavernous sinus, (c and d) postoperative contrast magnetic resonance imaging in axial and coronal images respectively showing very small residual tumor around the posterior cavernous ICA
Figure 4.
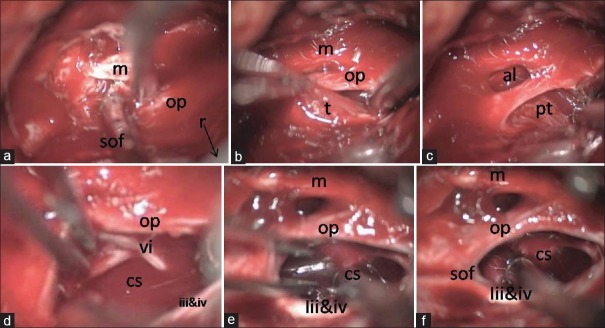
Peroperative sequential images of removal of right cavernous sinus hemangioma. (a) r – Retractor retracting temporal lobe with dura; m – Maxillary nerve; op – Opthalmic nerve and SOF – superior orbital fissure. (b) m –Maxillary nerve; op – Opthalmic nerve and t – Tumor. (c) al – anterio-lateral triangle and pt – Tumour removal through Parkinson's triangle. (d) After partial removal vi nerve identified dissecyed and preserved; op – opthalmic nerve; cavernous sinus – cavernous sinus; vi – abducent nerve and iii and iv – oculomotor and trochlear nerve. (e) Tumor near totally removed. m – Maxillary nerve; op – opthalmic nerve, cavernous sinus – cavernous sinus and iii and iv – oculomotor and trochlear nerve. (f) After complete tumor removal. m – Maxillary nerve; op – opthalmic nerve; cavernous sinus – cavernous sinus; SOF – superior orbital fissure and iii and iv – oculomotor and trochlear nerve
New postoperative 3rd nerve palsy developed in 5 cases that recovered completely in all cases except one where it recovered incompletely. Four patients had preoperative 3rd nerve palsy; three recovered completely and one recovered incompletely after operation. In rest three cases, there was no pre- or post-operative 3rd nerve palsy.
In 5 cases, postoperative 4th nerve palsy developed where 4 recovered and one did not. There was 3 preoperative 6th nerve palsy where only one recovered postoperatively. In 7 patients, 6th nerve palsy developed after operation; only 1 recovered partially and rest of the cases are living with complete 6th nerve palsy. No patient had preoperative opthalmic nerve dysfunction. In 1 case temporary neuropathic keratitis developed due to opthalmic nerve neuropathy and that was managed conservatively. Postoperatively simultaneous 3rd, 4th and 6th nerve palsy developed in 4 cases where 3rd and 4th nerve recovered in all cases but 6th nerve palsy persisted in all cases. Annoying, irritating and intractable feeling of tickling down of warm water under the skin of left sided forehead was found in the patient of inflammatory disease of CS (Tolosa Hunt Syndrome) in early postoperative period and it disappeared gradually over the next 2 months.
Visual impairment was in 2 cases where 1 case recovered after surgery and the other case remain static as preoperative state.
Total resection was done in 9 cases. Very small residual tumor was present in 1 case and small residual tumor in 2 cases.
There was no perioperative mortality in this series. Lesions where there was residual tumor, the residual part of tumor did not increase in size till last follow-up with imaging. There was no recurrence (clinical or radiological) of lesion in any case of the series till last follow-up.
Representative cases
Case 1
A 36-year-old housewife presented [Figures 1 and 2] with recurrent left sided headache with eye ache, recurrent diplopia specially when looking toward to left side and severe visual impairment. She had no history of unconsciousness, vomiting or seizure. Her neurological and other systemic examination was absolutely normal during presentation except optic nerve. Her visual acuity was 6/36 and 6/24 on left and right eye respectively. There was signs bilateral optic atrophy. Severe bilateral visual fields impairment (left >right) on perimetry. Visual evoked potential reported optic neuropathy with axonal loss. MRI of brain revealed, a hyper intense on T1- and T2-weighted images lesion measuring 3 cm × 3 cm × 2.5 cm in left CS that intensified more after contrast administration.
Figure 2.
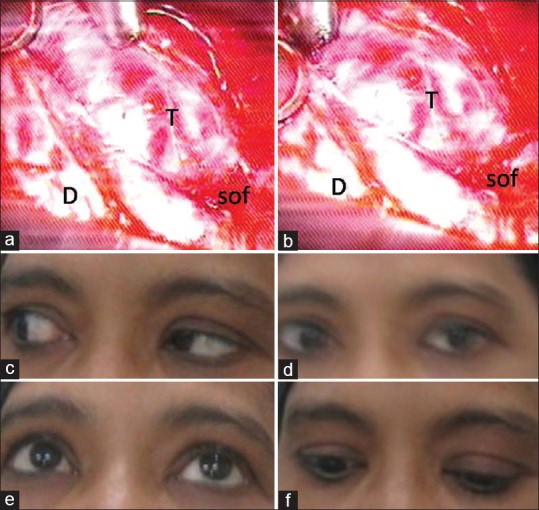
(a and b) Peroperative pictures of tumor of the patient of Figure 3 after exposure of tumor in cavernous sinus through middle cerebral fossa extradural approach. T – Tumor; D – temporal dura, SOF – superior orbital fissure. (c-f) Postoperative pictures of ocular movements showing persisting paresis of left 6th nerve 3 months after operation that resolved after 6th month of operation
Operation
Under general anesthesia with endotracheal intubation patient was placed on lateral position by keeping left side up. Head side of the table was elevated 30° with 15° right lateral flexion of neck. A lumbar subarachnoid drain was inserted to drain CSF for brain relaxation. A linear incision starting from the front of tragus and ending at midline 2 cm behind the coronal suture was made. Fascia and temporal muscle were splitted in the incision line and dissected subperiostealy and retracted with self-retaining mastoid retractor. A 5 cm × 5 cm temporal craniotomy was done behind the pterional point. Dura was separated from the temporal bone downward toward the base and bone was excised under the temporalis muscle after subperiosteal dissection up to temporal base under microscopic vision. Foramen spinosum was identified middle meningeal vessel was coagulated and cut. Then temporal basal dura was stripped up from lateral wall of CS and then mandibular nerve, maxillary, trigeminal ganglion were identified. With further dural striping opthalmic, trochlear and oculomotor nerves were identified near the SOF. Dura with temporal lobe was retracted and elevated with self-retaining table mounted retractor. The red, very vascular, spongy and compressible mass was identified between maxillary and opthalmic nerves. Tumor was removed partially through anterio-lateral triangle. Then the tumor was removed through the Parkinson's triangle. During tumor removal 6th nerve was carefully preserved from Dorello's canal to SOF. During separation of tumor from internal carotid artery (ICA) infero-lateral trunk (ILT) was avulsed with bleeding from ICA which was controlled with repeated short burst bipolar diathermy coagulation. There was huge bleeding from the opening of superior opthalmic vein, superior and inferior inter CS as well as superior and inferior petrosal sinus which were controlled by spongstan and surgicel packing. After complete tumor removal hemostasis was achieved with patience. Free bone flap was kept in position and wound closed in layers. During operation four units of blood transfusion was needed. Postoperatively patient developed complete ptosis with opthalmoplegia that recovered completely by 10 weeks after operation except abducent nerve. Abducent nerve recovered incompletely with some persistent paresis. Postoperatively her vision remains static as preoperative state. Histopathological examination confirmed CS hemangioma (CSH). Postoperative MRI of brain 8 months after operation showed near total resection of tumor (very small residual tumor posteriorly around the ICA). Patient remained neurologically static for last 21 months (last follow-up).
Case 5
A 39-year-old male computer operator presented [Figure 3] with progressively increasing headache, eye ache, left sided visual disturbance, diplopia and finally left sided ptosis. He had no history of unconsciousness, vomiting or seizure. His left sided visual acuity reduced to finger count (after manual elevation of upper eyelid) and on right side it was 6/6. There was complete opthalmoplegia (3rd, 4th and 6th nerve palsy) with ptosis on left side. Other physical examination revealed no abnormality. Perimetry showed left nasal field and partially temporal field visual impairment. MRI of brain T1-weighted and T2-weighted images showed an iso intense 3 cm × 2.5 cm × 2.5 cm lesion in left CS which intensified abruptly after contrast injection. After left sided temporal craniotomy, through subtemporal extradural (STExD) approach (as described in case 1) tumor was removed completely by separating it from ICA and 6th nerve was partially injured. Partially invaded and destroyed anterior clinoid process (ACP) came out with the tumor. Surprisingly there was minimum and easily controllable. Peroperatively two units of blood transfusion were required. Postoperatively patient remained in preoperative state of complete ptosis with opthalmoplegia for 8 weeks after operation. Then ptosis and other 3rd nerve function recovered incompletely within next 12 weeks. 4th and 6th nerve functions remained as before. Vision recovered to normal within 12 weeks after operation. Histopathological examination reported osteoclastoma. Though we requested review of the histopathological slides but report was the same. Postoperative MRI of brain 6 months after operation showed complete resection of tumor. Patient was tumor free with static neuro-deficit till last follow-up (2 years).
Figure 3.
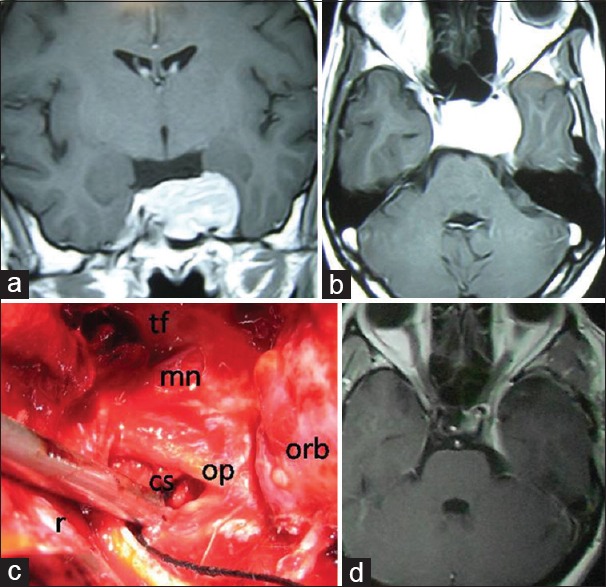
Preoperative contrast magnetic resonance imaging of brain. (a) Coronal and (b) axial images showing contrast enhancing tumor in left cavernous sinus (postoperative histopathology-osteoclastoma). (c) Per operative picture of left cavernous sinus after tumor removal from left cavernous sinus. tf – Temporal floor; r – retractor retracting temporal lobe with dura; mn – mandibular nerve; op – opthalmic nerve; cavernous sinus – cavernous sinus and orb – orbital content within periorbita. (d) Postoperative contrast magnetic resonance imaging of brain (9 months after operation) showing no residual or recurrent tumor
Discussion
The CS contains vital neurovascular structures that may be affected by vascular, neoplastic, infective, and infiltrative lesions arising in the CS proper or via extension from adjacent intra- and extra-cranial regions. Common lesions involving the CS are neoplastic, inflammatory, infective, granulomatus and vascular ones. The most common are neurogenic tumors and hemangioma. Tumors of the nasopharynx, skull base, and sphenoid sinus may extend to the CS through perineural and hematogenous metastases.[11] In the literature there is no report on CS osteoclastoma and nonneoplastic lymphatic aggregation in the literature. Surprisingly and interestingly we found such cases in our series. In the Tolosa–Hunt syndrome case of our series, the patient was suffering intractable headache, nonresponsive to steroid and analgesic. After surgery we put him on inject able methyle prednisolone for 7 days and then oral dexamethasone for another 6 weeks. In this patient, postoperative complaint of feeling of tickling down of warm water under the skin of left sided forehead was found. Cause of this symptom is not known. It was irritable, disturbing and intractable. It may be due to surgical manipulative stimulation of some special sensation bearing nerve fibers of opthalmic nerve. Tolosa–Hunt syndrome is a term applied to a retro-orbital pseudo tumor extending to the CS. Its clinical triad includes unilateral ophthalmoplegia, cranial nerve palsies, and a dramatic response to systemic corticosteroids. The process is usually unilateral but may be bilateral (5%).[12,13,14] MRI images may confuse with meningioma, tuberculoma, sarcoidosis, plasma cell granuloma, idiopathic hypertrophic cranial pachymeningitis etc., When diagnosis in doubt surgical excisional biopsy may be needed. As with metastases, lymphoma and leukemia reach the CS by direct extension from a primary lesion or from hematogenous spread.[15,16] There is several report on lymphocytic hypophsitis, infundibulo-hypositis where there may be panhypopituitarism, diabetes insipidus may occur.[17,18] But patient with nonneoplastic lymphoid infiltration only in CS with 6th nerve palsy and retro-orbital pain and without ICA involvement was probably not reported in the literature. The cells in this lesion were CD3 and CD20 negative but CD10 positive. Slight female predominant giant cell tumors comprise approximately 5% of all skeletal tumors. Peak incidence occurs during the second through fourth decades.[19] Giant cell tumors usually arise at the ends of long bones. Very rarely it occurs in skull with preponderance to sphenoid bone. Local bony destruction causing neurologic deficit is more common when these tumors arise in the medial sphenoid bone near the CS because of proximity to vital structures. One case of acute visual loss has been reported because of extension of a lesion centered on the ACP.[20] Radio graphically, the lesions are radiolucent with a nonsclerotic border. Treatment is surgical excision. Role of radio and chemotherapy is controversial.[19,20,21] In our case preoperatively, possibility of bony tumor in CS from ACP was not thought. Peroperatively, destroyed and invaded ACP that came out with the tumor brought some suspicion on the possibility of bony tumor. Postoperatively when histopathology reported osteoclastoma, we went for reconfirmation.
Three primary surgical approaches to the CS are usually used: The cranio-orbitozygomatic (COZ) approach, the extended middle cranial fossa zygomatic approach and middle cranial fossa STExD (MCF-STExD) approach.[3] MCF-STExD approach was used in 9 cases and in two cases extended middle fossa zygomatic approach. In one case, after fronto-temporal craniotomy, middle fossa-STExD approach used along with enlargement of SOF by cutting its bony margins to remove retro-orbital part of tumor. COZ approach was not used in any case. Except CSHs, peroperative bleeding in CS lesion was not too much and was easily controllable. Peroperatively in two cases (one meningioma and one hemangioma) meningohypophyseal artery was avulsed from ICA and in another two cases (one meningioma and one hemangioma) ILT was avulsed from ICA. In all four cases bleeding from ICA was controlled by patient cotton patty pressure and repeated short low bipolar diathermy burst. Here in three cases three units blood transfusion needed and one case four units blood transfusion needed. Venous bleeding is more in CSH due to retrograde flow of connecting venous sinuses (superior opthalmic vein, superior and inferior inter CSs and superior and inferior petrosal sinuses). All these venous connections needed surgicel packing for control of retrograde venous bleeding. In the rest of the cases, peroperative bleeding was minimum to moderate and blood transfusion needed 0–2 units. There was no catastrophic partial or complete rupture of ICA in any case during tumor dissection. In 6th nerve schwannomas in CS peroperative bleeding from operative site was 50–200 ml. 3rd, 4th and opthalmic nerves were identified and preserved anatomically in all cases. 6th cranial nerves was identified in all cases and anatomically preserved in 10 cases. In one case it was totally amalgamated with the tumor and anatomical preservation was not possible though this nerve was nonfunctioning till patient's childhood. In another case abducent nerve was partially injured during tumor dissection.
Patients with CS lesion usually present with CS syndrome.[11] Headache, cranial neuropathies (especially III, IV, V/II, V/III, VI), worsened vision and acuity, diplopia, ptosis, retro-orbital pain, exophthalmos, endocrinopathy, trigeminal neuralgia, hemi- or mono paresis can be seen in these patients.[22] In our series common clinical features were headache, eye ache, retro-orbital pain, diplopia, ptosis, opthalmoplegia with 3rd, 4th and 6th nerve palsy in isolated or in combination form. Here, visual impairment was in two cases and proptosis was in one case. The clinical and radiological findings are essential for deciding therapeutic modalities such as microsurgery, radiation therapy, or medical treatment as well as for appropriate planning of surgery or radiation therapy.[11,23,24,25] Schwannomas may arise from III, IV, VI cranial nerves[11,23] and carotid nerve plexus[6] in the CS, particularly cranial nerve III. Schwannoma arising from oculomotor, trochlear and abducent nerves are extremely rare. In our series surprisingly, we got three cases of 6th nerve schwannomas but no 3rd or 4th nerve schwannoma. Multiple CS schwannomas and bilateral acoustic ones are seen in patients with neurofibromatosis type 2.[11,26] Its imaging findings are nonspecific, and the diagnosis is made by postoperative histology. Here again the main problem is postoperative nerve palsy.[11] We found, the cavernous ICA was displaced inferiorly and medially in all three CS abducent nerve schwannoma. CSH is among the most common primary CS tumors along with schwannoma and meningioma.[11] The blood supply of CSH usually comes from branches of the intracavernous ICA (meningohypophyseal trunk/ILT or both) or external carotid artery via the middle meningeal or accessory meningeal arteries. CSH are well-demarcated by the presence of a fibrous pseudocapsule as a dissection plane. Total removal of these lesions is difficult due to the risk of severe intraoperative bleeding and the complicated neurovascular structures of the CS. Oculomotor, trochlear and trigeminal nerves can be separated from the lesion by their dural sleeves but separation of the abducent nerve is difficult as it is not protected by a dural sheath. Only a few reported cases have been totally removed without cranial nerve injury.[22,27,28,29] We found total or near total excision of CSH is not technically difficult but there is every chance of hypovolumia from bleeding and identification and preservation of 6th nerve is technically demanding but possible. Anatomical preservation does not mean physiological preservation. In two of our cases permanent 6th nerve palsy (one preoperative and one postoperative) occurred. Induced hypotension or preoperative embolization may also help to control the bleeding during surgery but we think it is not necessary. Radiation therapy has been recommended as a primary treatment or adjuvant therapy to decrease the size and vascularity of the mass. Stereotaxic radiotherapy or gamma knife can be performed to the rest of the tumor pieces or if the surgical excision is impossible.[22,27,28,29] Most CS meningiomas arise from the lateral dural wall, but sometimes they may be exclusively inside the CS. But true CS meningioma is very rare. Meningioma may constrict the lumen of the ICA. They may have an appearance very similar to schwannomas.[3,11] CS meningiomas can be very difficult to treat. It is also critical that these patients understand the natural history, management options, and risks associated with every treatment. Further, it is critical to understand the patient's goals of treatment. Currently, microsurgical resection of CS meningioma stands alone as the only treatment associated with total tumor removal. No other treatment can achieve total removal or disappearance consistently. But the main problem of total resection is cranial neuropathy. The majority of neuropathies in these patients exist at presentation, whereas a minority develops permanently after surgery, and many of those that occur affect sensory function or can be treated with strabismus surgery. Optic nerve deficits are infrequently encountered. Multiple surgical centers across the world have extensive expertise at treating such tumors, and techniques and outcomes continue to evolve.[3] We also faced postoperative multiple cranial nerves palsy with persistent 6th nerve palsy (where 3rd and 4th nerve recovered) in both cases of CS meningioma. An epidermoid cyst may be of extracavernous origin and extend into the CS, originate in the lateral CS wall (interdural cyst), or be a true intracavernous lesion.[29,30,31] CS dermoid is the differential diagnosis of CS epidermoid.[11,32] Surgical excision is the treatment of choice and usually curative. Tuberculosis is, in some parts of the world, a relatively common.[33,34] As tuberculosis is a common disease in our part of the earth we were not surprised by the report of tuberculosis from a lesion from CS.
By any means, our series is not comparable to other series like Cusimano et al.[6] and Pamir et al.[35] series. Even though, our numbers of cases are very small, we did not face any seriously disabling morbidity other than cranial nerve palsy. We did not face cerebral infarction, CSF fistula or hematoma. Complete excision rate is 9 (70.5%) out of 12 cases. There was no mortality or recurrence.
Conclusion
Benign lesion interior to the CS are very rare but they are not so rare for a practicing skull base surgeon. Though decision for microsurgical removal of such lesions is not straight forward and should come after considering all aspects of patient and lesion. Probably microsurgery is the best option in treating such benign lesions though it may associate with permanent cranial nerve palsy (especially 6th nerve palsy). Finally it can be concluded that benign lesion inside the CS can be excised micro surgically with relative safety.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chowdhury F, Haque M, Kawsar K, Ara S, Mohammod Q, Sarker M, et al. Transcranial microsurgical and endoscopic endonasal cavernous sinus (CS) anatomy: A cadaveric study. J Neurol Surg A Cent Eur Neurosurg. 2012;73:296–306. doi: 10.1055/s-0032-1322519. [DOI] [PubMed] [Google Scholar]
- 2.Parkinson D. A surgical approach to the cavernous portion of the carotid artery. Anatomical studies and case report. J Neurosurg. 1965;23:474–83. doi: 10.3171/jns.1965.23.5.0474. [DOI] [PubMed] [Google Scholar]
- 3.Heth JA, Al-Mefty O. Cavernous sinus meningiomas. Neurosurg Focus. 2003;14:e3. doi: 10.3171/foc.2003.14.6.3. [DOI] [PubMed] [Google Scholar]
- 4.Rhoton AL, Jr, Hardy DG, Chambers SM. Microsurgical anatomy and dissection of the sphenoid bone, cavernous sinus and sellar region. Surg Neurol. 1979;12:63–104. [PubMed] [Google Scholar]
- 5.Sekhar LN, Burgess J, Akin O. Anatomical study of the cavernous sinus emphasizing operative approaches and related vascular and neural reconstruction. Neurosurgery. 1987;21:806–16. doi: 10.1227/00006123-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cusimano MD, Sekhar LN, Sen CN, Pomonis S, Wright DC, Biglan AW, et al. The results of surgery for benign tumors of the cavernous sinus. Neurosurgery. 1995;37:1–9. doi: 10.1227/00006123-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983;58:824–31. doi: 10.3171/jns.1983.58.6.0824. [DOI] [PubMed] [Google Scholar]
- 8.Dolenc VV, Kregar T, Ferluga M, Fettich M, Morina A. Treatment of tumors invading the cavernous sinus. In: Dolenc VV, editor. The Cavernous Sinus. A Multidisciplinary Approach to Vascular and Tumorous Lesions. New York: Springer-Verlag; 1987. pp. 377–91. [Google Scholar]
- 9.Fukushima T, Day JD. Surgical management of tumors involving the cavernous sinus. In: Schmidek HH, Sweet WH, editors. Operative Neurosurgical Techniques. Philadelphia: W. B. Saunders; 1995. pp. 493–510. [Google Scholar]
- 10.Hakuba A, Matsouka Y, Suzuki T, Komiyama M, Jin TB, Inoue Y. Direct approaches to vascular lesions in the cavernous sinus via the medial triangle. In: Dolenc VV, editor. The Cavernous Sinus. A Multidisciplinary Approach to Vascular and Tumorous Lesions. New York: Springer-Verlag; 1987. pp. 272–84. [Google Scholar]
- 11.Razek AA, Castillo M. Imaging lesions of the cavernous sinus. AJNR Am J Neuroradiol. 2009;30:444–52. doi: 10.3174/ajnr.A1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cakirer S. MRI findings in the patients with the presumptive clinical diagnosis of Tolosa-Hunt syndrome. Eur Radiol. 2003;13:17–28. doi: 10.1007/s00330-002-1458-3. [DOI] [PubMed] [Google Scholar]
- 13.Calistri V, Mostardini C, Pantano P, Pierallini A, Colonnese C, Caramia F. Tolosa-Hunt syndrome in a patient with systemic lupus erythematosus. Eur Radiol. 2002;12:341–4. doi: 10.1007/s003300100960. [DOI] [PubMed] [Google Scholar]
- 14.de Arcaya AA, Cerezal L, Canga A, Polo JM, Berciano J, Pascual J. Neuroimaging diagnosis of Tolosa-Hunt syndrome: MRI contribution. Headache. 1999;39:321–5. doi: 10.1046/j.1526-4610.1999.3905321.x. [DOI] [PubMed] [Google Scholar]
- 15.Huisman TA, Tschirch F, Schneider JF, Niggli F, Martin-Fiori E, Willi UV. Burkitt's lymphoma with bilateral cavernous sinus and mediastinal involvement in a child. Pediatr Radiol. 2003;33:719–21. doi: 10.1007/s00247-003-1010-x. [DOI] [PubMed] [Google Scholar]
- 16.Karadag D, Karagülle AT, Erden I, Erden A. Trigeminal nerve involvement in T-cell acute lymphoblastic leukemia: Value of MR imaging. Eur J Radiol. 2002;44:16–8. doi: 10.1016/s0720-048x(01)00388-6. [DOI] [PubMed] [Google Scholar]
- 17.Kartal I, Yarman S, Tanakol R, Bilgic B. Lymphocytic panhypophysitis in a young man with involvement of the cavernous sinus and clivus. Pituitary. 2007;10:75–80. doi: 10.1007/s11102-007-0003-4. [DOI] [PubMed] [Google Scholar]
- 18.Huang CH, Chou KJ, Lee PT, Chen CL, Chung HM, Fang HC. A case of lymphocytic hypophysitis with masked diabetes insipidus unveiled by glucocorticoid replacement. Am J Kidney Dis. 2005;45:197–200. doi: 10.1053/j.ajkd.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Mirra JM. Giant cell tumor (osteoclastoma). Bone Tumors. Philadelphia: Lea and Febiger; 1989. pp. 119–40. [Google Scholar]
- 20.Ilangovan S, Cuison R, Harper T, Alvarez A, Pannu Y, Ilangovan C. Giant cell tumor of the skull base with acute vision loss. J Neuropathol Exp Neurol. 2002;61:476. [Google Scholar]
- 21.Harris AE, Beckner ME, Barnes L, Kassam A, Horowitz M. Giant cell tumor of the skull: A case report and review of the literature. Surg Neurol. 2004;61:274–7. doi: 10.1016/S0090-3019(03)00428-2. [DOI] [PubMed] [Google Scholar]
- 22.Cozar M, Iplikcioglu AC, Gokduman CA, Bek S, Hatipoglu MA. Cavernous sinus hemangiomas: Two case reports. Turk Neurosurg. 2005;15:123–8. [Google Scholar]
- 23.Boardman JF, Rothfus WE, Dulai HS. Lesions and pseudolesions of the cavernous sinus and petrous apex. Otolaryngol Clin North Am. 2008;41:195–213, vii. doi: 10.1016/j.otc.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Lee HK, Park JK, Choi CG, Suh DC. Cavernous sinus syndrome: Clinical features and differential diagnosis with MR imaging. AJR Am J Roentgenol. 2003;181:583–90. doi: 10.2214/ajr.181.2.1810583. [DOI] [PubMed] [Google Scholar]
- 25.Yagi A, Sato N, Taketomi A, Nakajima T, Morita H, Koyama Y, et al. Normal cranial nerves in the cavernous sinuses: Contrast-enhanced three-dimensional constructive interference in the steady state MR imaging. AJNR Am J Neuroradiol. 2005;26:946–50. [PMC free article] [PubMed] [Google Scholar]
- 26.Lo PA, Harper CG, Besser M. Intracavernous schwannoma of the abducens nerve: A review of the clinical features, radiology and pathology of an unusual case. J Clin Neurosci. 2001;8:357–60. doi: 10.1054/jocn.2000.0846. [DOI] [PubMed] [Google Scholar]
- 27.Peker S, Kiliç T, Sengöz M, Pamir MN. Radiosurgical treatment of cavernous sinus cavernous haemangiomas. Acta Neurochir (Wien) 2004;146:337–41. doi: 10.1007/s00701-004-0231-6. [DOI] [PubMed] [Google Scholar]
- 28.Shi J, Hang C, Pan Y, Liu C, Zhang Z. Cavernous hemangiomas in the cavernous sinus. Neurosurgery. 1999;45:1308–13. doi: 10.1097/00006123-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Bonde V, Goel A. Interdural cavernous sinus epidermoid cyst. J Clin Neurosci. 2008;15:212–4. doi: 10.1016/j.jocn.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Tatagiba M, Iaconetta G, Samii M. Epidermoid cyst of the cavernous sinus: Clinical features, pathogenesis and treatment. Br J Neurosurg. 2000;14:571–5. doi: 10.1080/02688690050206747. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Ikawa F, Kurisu K, Arita K, Takaba J, Kanou Y. Quantitative MR evaluation of intracranial epidermoid tumors by fast fluid-attenuated inversion recovery imaging and echo-planar diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001;22:1089–96. [PMC free article] [PubMed] [Google Scholar]
- 32.Tun K, Celikmez RC, Okutan O, Gurcan O, Beskonakli E. Dermoid tumour of the lateral wall of the cavernous sinus. J Clin Neurosci. 2008;15:820–3. doi: 10.1016/j.jocn.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Morgado C, Ruivo N. Imaging meningo-encephalic tuberculosis. Eur J Radiol. 2005;55:188–92. doi: 10.1016/j.ejrad.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Wilson JD, Castillo M. Magnetic resonance imaging of granulomatous inflammations: Sarcoidosis and tuberculosis. Top Magn Reson Imaging. 1994;6:32–40. [PubMed] [Google Scholar]
- 35.Pamir MN, Kilic T, Ozek MM, Ozduman K, Türe U. Non-meningeal tumours of the cavernous sinus: A surgical analysis. J Clin Neurosci. 2006;13:626–35. doi: 10.1016/j.jocn.2006.04.004. [DOI] [PubMed] [Google Scholar]