Figure 1. An optimum in complete amphitely fraction requires weak kinase relative to phosphatase activity in a single site phosphorylation model.
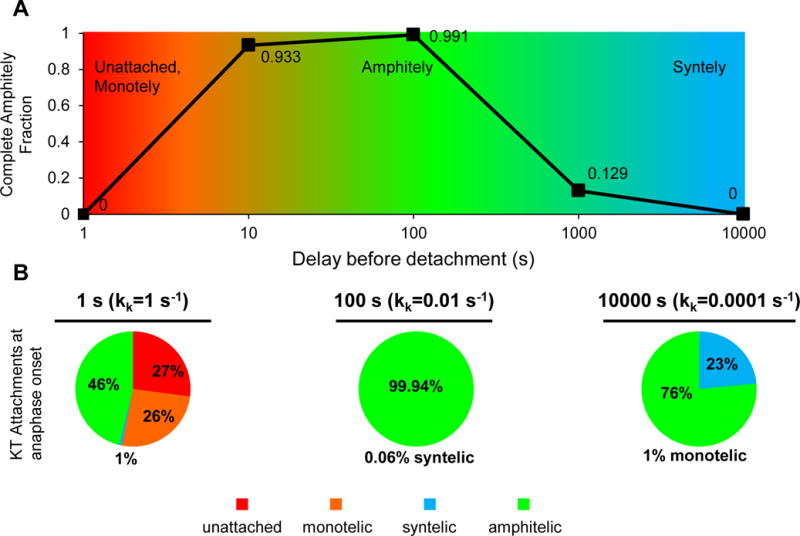
(A) Reducing the kinase rate relative to the phosphatase rate in the single site phosphorylation model results in increased delays before detachment and the emergence of an optimum in the complete amphitely fraction, i.e. fraction of spindles with all amphitelic kinetochore pairs at anaphase onset (n=1000 simulated spindles for each phosphatase/kinase ratio with delays before detachment of 1–10000 s, X2=4000, df=4, p<10−100).
(B) Distribution of kinetochore attachment states at anaphase onset. For a 1 s delay before detachment, unattached kinetochores and monotelic attachments were both high because detachment was too fast. For a 100 s delay, amphitelic attachments reached nearly 100% because the delay before detachment was sufficient to solve the IPBO. For a 10000 s delay, syntelic attachments were high because the delay before detachment was too long, (X2=12000, df=2, p<10−100 (amphitelic versus not amphitelic)).
