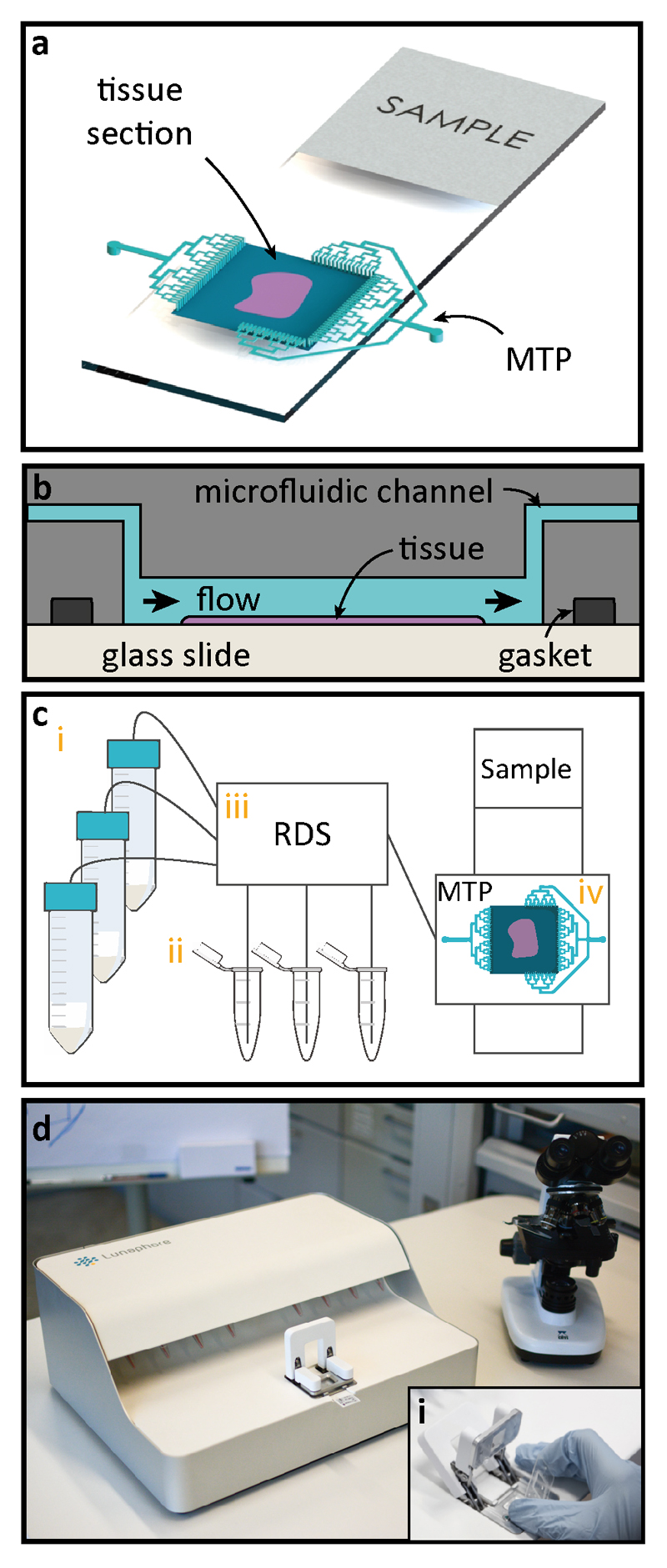
Fig. 1. Working principle of microfluidic staining of FS.
(a) Once the frozen sample preparation is completed, the sample is clamped using a gasket to the Microfluidic Tissue Processor (MTP) to form a reaction chamber of 17x17 mm2, after which the system is ready for fast IHC staining. Microfluidic channels deliver reagents to and from the reaction chamber. Staining takes place only in the chamber (b) Schematic cross-section of the MTP clamped to the tissue slide. Inside the reaction chamber, reagents are sequentially delivered and washed over the surface of the tissue section. The MTP enables a fast fluidic exchange on a one-second timescale, while keeping reagent exposure times uniform throughout the whole tissue surface. (c) Schematics of the laboratory setup used for the experiments: (i) 50 mL reservoirs are used for common reagents, namely PBS, DIW, DAB, and hematoxylin; (ii) 1.5 mL Eppendorf tubes are used to load specific reagents, such as antibodies, and blocking solutions; (iii) a computer-operated valve-manifold called Reagent Delivery System (RDS) allows for the selection of the reagents that are flushed into the chamber of reaction; (iv) MTP/ tissue sample staining chamber. (d) Picture of the prototype used for the experiments. The inset (i) shows the mechanism of manual insertion of the MTP in the machine.