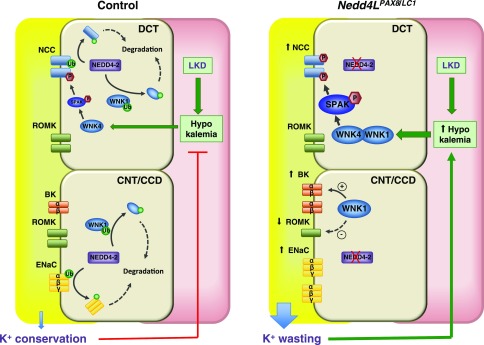
Figure 8.
Model of NEDD4-2 action in K+ conservation in the CNT/CCD. Under K+ restriction, the aldosterone/MR/SGK1 regulatory axis is switched off, resulting in maximal NEDD4-2 activity. In controls, the active NEDD4-2 primarily downregulates ENaC. WNK1 is also downregulated, resulting in limited BK channel activity, Moreover, hypokalemia causes WNK4 activation, and stimulation of SPAK and NCC to limit distal Na+ delivery to ENaC and voltage-dependent K+ excretion via ROMK. Collectively, these transport processes act synergistically to promote K+ conservation. The deregulation of this process in Nedd4LPax8/LC1 mice results in K+ wasting, primarily because of the release of ENaC from tonic NEDD4-2 inhibition. WNK1 is also increased, causing elevated BK expression. The severe hypokalemia caused by enhanced K+ wasting further stimulates WNK1/WNK4, SPAK and NCC, which, together with reduced ROMK expression, partially compensates for the K+ wasting induced by NEDD4-2 deletion.