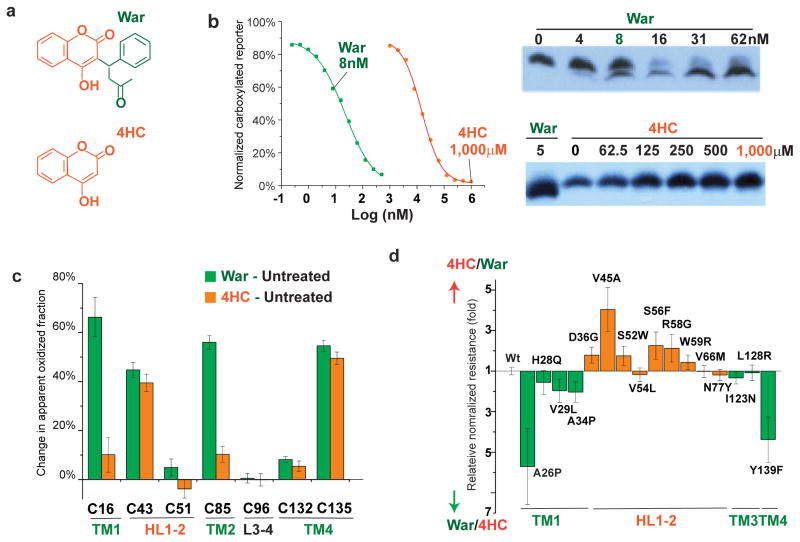
Figure 6. The orientation of warfarin binding to hVKOR.
(a) Chemical structures of warfarin (War) and 4-hydroxycoumarin (4HC), with the 4HC and the warfarin side group shown in orange and green, respectively. (b) Left, Inhibition of hVKOR activity by warfarin and 4HC. Right, 4HC does not induce the gel shift that is associated with warfarin binding. (c) Cysteine footprinting shows that the side group of warfarin interacts with the TMs of hVKOR. Each bar shows the difference in NEM alkylation at specific cysteine residues induced by adding warfarin or 4HC to cells expressing hVKOR (i.e. subtracting untreated from treated for each cysteine). Warfarin significantly changes the alkylation of four cysteines. In contrast, without the warfarin side group, 4HC has much less effect on Cys16 in TM1 and Cys85 in TM2. Error bars, same analysis as in Fig. 2b but with error propagation calculated for the subtraction. (d) Comparison of normalized resistance of hVKOR mutants to 4HC and to warfarin. WRs on TMs (green) and HL1-2 (orange) show stronger resistance to warfarin and 4HC, respectively. The calculation of relative normalized resistance and s.e.m. is described in detail in Online Methods. Uncropped blot/gel images are shown in Supplementary Data Set 2. Source data for resistance and basal activity are available in Supplementary Data Set 3, 4.