Abstract
Background and Objectives
The role of hyperthermic intraperitoneal chemoperfusion (HIPEC) in the multimodality treatment of ovarian peritoneal metastases (OPM) and primary peritoneal cancer (PPC) remains controversial. We hypothesized that cytoreductive surgery (CRS) and HIPEC would provide meaningful survival benefit without excessive morbidity.
Methods
We reviewed clinicopathologic and perioperative data following 96 CRS-HIPEC procedures for primary or recurrent OPM and PPC. Kaplan-Meier survival curves and multivariate Cox-regression models identified prognostic factors affecting oncologic outcomes.
Results
CRS-HIPEC was mostly performed for recurrent disease (56.3%) and high-grade serous carcinoma (72.9%). Platinum-based systemic chemotherapy was administered to 89.5% of patients, with 75.5% having platinum-sensitive disease at CRS-HIPEC. Complete macroscopic resection was achieved in 70.8% of patients. Clavien-Dindo grade 3/4 morbidity occurred in 23.4% of patients; 3 patients died within 60-days postoperatively. Median overall survival from diagnosis of peritoneal metastases and CRS-HIPEC was 78 and 38 months, respectively. Completeness of cytoreduction, pathologic subtype and 30-day morbidity were independent predictors of survival in multiple regression analysis.
Conclusions
Our study demonstrates promising survival data and supports the role of HIPEC in the multimodality treatment algorithm for primary or recurrent OPM and PPC. However definite indications and timing of HIPEC need to be clarified by prospective studies.
Keywords: Ovarian cancer, Primary peritoneal cancer, Cytoreductive surgery, HIPEC multimodality, cisplatin
INTRODUCTION
The majority of patients with ovarian cancer (approximately 65–75%) present with advanced-stage disease (American Joint Committee on Cancer [AJCC] stages III and IV).(1) Metastatic disease confined to the peritoneal cavity (AJCC stage III) is the most common cause of treatment failure and death in patient with primary and recurrent ovarian cancer.(2, 3) For advanced primary ovarian cancer limited to the peritoneal cavity standard therapy includes maximal cytoreductive surgery (CRS) of macroscopic disease, followed by adjuvant intravenous (IV) chemotherapy (usually carboplatin plus paclitaxel).(4) This multimodality approach achieves median survival of 41–57 months, 5-year survival rates of 40–55% and long-term 10-year) survival rates of 20–25% following “optimal” cytoreduction (traditionally defined as < 1cm diameter residual after surgery).(4–8) However, percentage maximal cytoreduction is a major determinant of survival such that complete macroscopic resection has been shown to correlate with superior survival.(9)
The addition of intraperitoneal (IP) dwell chemotherapy to CRS and IV chemotherapy has been shown to delay recurrence in patients with “minimal residual disease” (< 1 cm diameter residual tumor nodules) after surgery, but more importantly it has been shown to increase long-term 10-year) cure rates in patients with “no residual macroscopic disease” after surgery. Data from randomized trials of IP dwell chemotherapy demonstrate median survival of 49–66 months and 5- and 10-year survival rates of 40–60% in patients with “no residual macroscopic disease” after surgery, while those with “minimal residual disease” achieve improved short-term survival (median survival 53 months and 5-year survival rate 45%), while 10-year survival remains lower (18%).(5–8, 10, 11) However, IP dwell chemotherapy has not been widely adopted due to complexity of administration, logistical issues, IP-catheter associated complications, poor patient compliance, and poor tolerance due to increased toxicity.(10, 12)
Given the challenges and lack of acceptance of IP dwell chemotherapy, hyperthermic intraperitoneal chemoperfusion (HIPEC) has been evaluated in advanced stage III ovarian cancer with the aim to eliminate residual microscopic disease and thereby reduce disease recurrence and prolong survival. HIPEC is now widely performed following maximal CRS to treat peritoneal metastases from appendix cancer, colorectal cancer and malignant peritoneal mesothelioma and this combination has demonstrated survival benefit.(13–15) The benefits of HIPEC over IP dwell chemotherapy include 1) single treatment at the time of surgery with high-dose chemotherapeutic agents (20–1000-fold higher drug concentration); 2) direct cytotoxicity of hyperthermia; 3) synergistic enhancement of chemotherapeutic effect by hyperthermia; and 4) intraoperative administration without the need for IP-catheter placement or postoperative IP treatments.(16, 17) CRS-HIPEC has been evaluated as first-line therapy for ovarian PM in several small prospective phase I-II studies and some retrospective case series. These data demonstrate median survival of 27–78 months (weighted mean 37.6 months) and 5-year survival rates of 28–72% (weighted mean 40%).(18–23) However, current consensus statements by international gynecologic cancer groups and the National Comprehensive Cancer Networks (NCCN) do not advocate for HIPEC as a standard therapeutic option for primary ovarian PM. A number of international randomized trials of CRS-HIPEC are currently ongoing to evaluate its utility in advanced primary or recurrent ovarian cancer.(19)
Locoregional ovarian cancer recurrence confined to the peritoneal cavity occurs in over 65% of patients within 5-years, despite excellent response rates to maximal front-line multimodality therapy.(6) Pooled analysis of multiple retrospective studies and a recent meta-analysis demonstrate mean weighted median overall post-recurrence survival time of 30 months following CRS for recurrent ovarian PM, with complete macroscopic resection identified as a major predictor of survival.(24, 25) IP dwell chemotherapy has not been studied in the setting of recurrent ovarian cancer. Published series of CRS-HIPEC for patients with recurrent platinum-sensitive ovarian PM report median survival of 23–62 months (weighted mean 36.5 months) and 5-year survival rates of 18–57%.(18–20, 22, 23, 26–28) Spiliotis and colleagues recently published the only randomized trial of CRS-HIPEC for recurrent ovarian PM and demonstrated improved median survival in the HIPEC-group (27 vs. 13 months).(29)
We conducted this study to assess perioperative and oncologic outcomes following CRS-HIPEC as front-line therapy or following disease recurrence in a large cohort of patients with AJCC stage III ovarian peritoneal metastases (OPM) and primary peritoneal cancer (PPC) treated at a single high-volume center.
MATERIALS AND METHODS
From a prospective database we reviewed clinicopathologic and perioperative data of 96 CRS-HIPEC procedures (93 patients) for ovarian peritoneal metastases (OPM) and primary peritoneal cancers (PPC) between 2002 and 2015. The study was approved by the University of Pittsburgh institutional review board, and all procedures were performed by surgeons with extensive experience in regional therapies. Patients were evaluated in our peritoneal surface malignancy clinic and discussed at our multidisciplinary tumor board to determine suitability for CRS-HIPEC. Intra-operatively, volume of disease was quantified by the peritoneal cancer index (PCI) as described by Sugarbaker; combining lesion size and tumor distribution in specific abdomino-pelvic regions.(30) Cytoreductive Surgery (CRS) was performed in accordance with techniques, described by Bao and Bartlett, to achieve complete cytoreduction. Completeness of cytoreduction (CC) score assessed the extent of residual disease at the end of surgical resection; CC-0: no visible residual disease, CC-1: residual tumors ≤ 2.5 mm, CC-2: residual tumors 2.5 mm to 2.5 cm; CC-3: residual tumors ≥ 2.5 cm.(31) A standard institutional protocol for HIPEC was initiated following CRS to achieve a target intraperitoneal tissue temperature of 42°C for 100 minutes. Patients received either Mitomycin C (40 mg) or Cisplatin at 175 mg/m2. Postoperative morbidity was classified according to the Dindo-Clavien grading system.(32) Patients were categorized based on the platinum-free interval (i.e. interval between completion of last platinum-based treatment for the primary cancer and detection of relapse); patients with recurrence occurring > 6 months after treatment were considered platinum-sensitive; between 1–6 months were platinum-resistant; and during treatment or within 1 month after treatment were platinum-refractory.(33) Indication for CRS-HIPEC was categorized as follows; initial treatment, if performed as the first surgical therapy for the primary (non-recurrent) disease; interval treatment, if performed after neoadjuvant IV chemotherapy for the primary (non-recurrent disease); consolidation treatment, if performed following initial CRS (with < 1cm residual disease) and adjuvant IV chemotherapy; secondary treatment, if performed following initial CRS (with > 1cm residual disease) and adjuvant IV chemotherapy; and recurrent treatment, if performed for recurrent disease.
Statistical analysis was performed using STATA-10 (College Station, TX). P-values <0.05 were considered significant. Overall survival (OS) was calculated from the date of diagnosis of peritoneal metastases (for patients with PPC the date of diagnosis of the primary cancer was also considered the date of diagnosis of peritoneal metastases) or from CRS-HIPEC to the date of death. If a patient did not experience death, they were censored at the time of their last follow-up. Time to progression was calculated from the date of CRS-HIPEC to the date of tumor recurrence. If a patient did not experience progression or recurrence, they were censored on the date of their last follow-up or death. When progression or recurrence records were missing, and cancer could not be ruled out as a cause for death, these patients were included as having disease progression. Survival times were estimated using the Kaplan-Meier method. One patient was lost to follow-up and excluded from survival analyses. Proportional hazards Cox regression was used to examine both univariate and multivariate associations with mortality and progression. This was performed for two cohorts; the entire study population and for the ovarian high-grade serous cancer subgroup. In the univariate analysis we analyzed the following covariates for their effect on survival, including time-interval from diagnosis to PM, time-interval from PM until CRS-HIPEC, indication for CRS-HIPEC (initial or consolidation or secondary or interval or recurrent treatment), synchronous or metachonous PM, platinum-sensitivity (sensitive or resistant or refractory disease), duration of platinum-sensitivity (> 12 months or 6–12 months or < 6 months), preoperative systemic chemotherapy, response to preoperative chemotherapy, PCI, CC-score, pathologic subtype and postoperative complications. All factors that were examined in univariate analysis were considered for entry into the model for multivariate analysis. Variables were selected for the final multivariate model based a combination of selection methods.
RESULTS
Clinical and treatment history prior to CRS-HIPEC at our institution
For patients with ovarian cancer, the majority had synchronous PM at the time of diagnosis (n=67, 76.1%), while the median time-interval between diagnosis of primary ovarian cancer and metachronous PM was 47.7 months (range 1.5–256.2 months). The median time-interval from diagnosis of OPM until CRS-HIPEC at our institution was 17.4 months (range 0.03–226.3 months).
Resection of the primary cancer (OPM and PPC) with or without tumor debulking had been performed previously in 74 patients (77.1%). Platinum-based chemotherapy was administered as part of initial treatment of the primary cancer in 85 patients (89.5%) and was not given to 7 patients with borderline mucinous tumors, 1 patient each with granulosa cell cancer and carcinosarcoma, and 2 patients with high-grade serous carcinoma. In addition, 14 patients (14.6%) received IP chemotherapy as part of initial treatment of the primary cancer. Data regarding platinum-sensitivity at the time of CRS-HIPEC was available for 49 patients; 37 patients (75.5%) had platinum-sensitive disease; 11 patients (22.5%) had platinum-resistant disease, and 1 patient (2%) had platinum-refractory disease.(Table 1) Multiple drug regimens were administered for residual or recurrent disease to 50 patients (52.1%) prior to undergoing CRS-HIPEC at our institution. One patient underwent 3 HIPEC procedures at our institution, another had two, while one patient had undergone CRS-HIPEC prior to being treated at our institution.
Table 1.
Clinicopathologic and perioperative characteristics at the time of CRS-HIPEC at our institution (n=96)
| Reason for CRS-HIPEC; n (%) | Recurrent disease | 54 (56.3) |
| Interval | 20 (20.8) | |
| Initial | 14 (14.6) | |
| Consolidation | 5 (5.2) | |
| Secondary | 3 (3.1) | |
| Pathologic subtype; n (%) | Ovarian high-grade serous carcinoma | 62 (64.6) |
| Ovarian low-grade serous neoplasm | 1 (1) | |
| Mucinous borderline neoplasm | 10 (10.4) | |
| Mucinous adenocarcinoma | 4 (4.2) | |
| Clear cell carcinoma | 1 (1) | |
| Endometrioid carcinoma | 1 (1) | |
| Carcinosarcoma | 3 (3.1) | |
| Granulosa cell tumor | 6 (6.3) | |
| Primary peritoneal cancer (high-grade serous) | 8 (8.3) | |
| Platinum-sensitivity at the time of CRS-HIPEC; n (%) (n=49) | Platinum-sensitive | 37 (75.5) |
| Platinum-resistant | 11 (22.5) | |
| Platinum-refractory | 1 (2) | |
| Peritoneal cancer index; median (range) | 11 (0–30) | |
| CC-score; n (%) | CC-0 | 68 (70.8) |
| CC-1 | 19 (19.8) | |
| CC-2 | 7 (7.3) | |
| CC-3 | 2 (2.1) | |
CRS-HIPEC: cytoreductive surgery-hyperthermic intraperitoneal chemoperfusion; CC: cytoreductive surgery
Perioperative and histopathologic characteristics associated with CRS-HIPEC at our institution
CRS-HIPEC was performed for recurrent disease in 54 patients (56.3%), as interval treatment following neoadjuvant chemotherapy for primary (non-recurrent) disease in 20 patients (20.8%), as initial treatment for primary (non-recurrent) disease without neoadjuvant chemotherapy in 14 patients (14.6%), as consolidation therapy in 5 patients (5.2%) and as secondary therapy in 3 patients (3.1%).(Table 1) Preoperative systemic chemotherapy (defined as chemotherapy given within 2 months prior to CRS-HIPEC for primary or recurrent disease was administered to 50 patients (52.1%), while postoperative systemic chemotherapy was administered to 58 patients (66.7%). Response to preoperative systemic chemotherapy (defined as improvement of imaging or CA125 level or symptoms) was evident in 25 patients (53.2%), where data was available. Eighty-eight (91.7%) CRS-HIPEC procedures were performed for OPM, while 8 (8.3%) were performed for PPC. All PPC were high-grade serous carcinomas (n=8), while pathologic subtypes of ovarian cancer included serous carcinoma (low-grade= 1, high-grade= 62), mucinous carcinoma (borderline tumor= 10, carcinoma= 4), endometrioid cancer (n=1), clear cell cancer (n=1), carcinosarcoma (n=3), and granulosa cell tumor (n=6).(Table 1)
The median age at the time of CRS-HIPEC at our institution was 57.2 years (range 25.8–84.4 years) and the median age-adjusted Charlson comorbidity index was 8 (range 6–14). Median PCI was 11 (range 0–30) and most patients had complete macroscopic resection (CC-0: 68; CC-1: 19, CC-2: 7, CC-3: 2 patients).(Table 1) The majority of patients received mitomycin C (81.3%) during HIPEC, while 18.8% received cisplatin. Clavien-Dindo morbidity (grades 1–4) occurred in 63 patients (66.3%) at 30 days, with major grade 3–4 morbidity occurring in 23.4% of patients. Clavien-Dindo grade 3–4 renal complications occurred in 3 patients (16.7%) perfused with cisplatin, compared to 1 patient (1.3%) perfused with mitomycin C. Postoperative mortality occurred in 4 patients (4.2%) at 90 days. Two deaths occurred in patients with high-grade serous carcinomas and one each with borderline mucinous tumor and mucinous adenocarcinoma.
Oncologic outcomes for patients undergoing CRS-HIPEC at our institution
The median estimated follow-up duration from CRS-HIPEC and diagnosis of PM for the study population was 89.6 and 114.6 months, respectively. At the time of analysis, 57 patients (61.9%) had died and 73 patients (86.9%) had progressed.
For the entire study population (n=96), the estimated median OS calculated from CRS-HIPEC was 38 months (95% CI 26.9, 53.7), while the estimated median OS calculated from diagnosis of PM was 77.8 months (95% CI 56.1, 94.4). The probability of survival at 1-, 3-, and 5-years calculated from CRS-HIPEC was 82%, 53% and 36%, respectively. The probability of survival at 1-, 3- and 5-years calculated from diagnosis of PM was 97%, 76% and 57%. The estimated median PFS calculated from CRS-HIPEC was 13.3 months (95% CI 9.6, 19.9). The probability of PFS at 1-, 3-, and 5-years calculated from CRS-HIPEC was 52%, 32% and 6%, respectively. Multiple regression analysis for OS in the entire patient population of this study demonstrated CC-score (p<0.001), pathology (p=0.001) and morbidity at 30 days (p=0.01) to be independent predictors.(Table 2) The estimated median OS by completion of cytoreduction was 41.8 months (95% CI 27.4, 68.9) for CC-0 resection; 31.3 months (95% CI 11.1, −) for CC-1 resection; 34.4 months (95% CI 1, 56.2) for CC-2 resection; and 6.5 months (95% CI 3.9, 9.1) for CC-3 resection. The estimated median OS for patients without morbidity at 30 days was 50 months (95% CI 29.4, 104.6) and for those with morbidity was 35.5 months (95% CI 22.3, 53.7). The estimated median OS stratified by pathology was not reached for mucinous borderline tumor, low-grade serous carcinoma and granulosa cell carcinoma, while survival was 69.8 (95% CI −) months for endometrioid carcinoma, 39.2 months (95% CI 1.9, −) for mucinous carcinoma, 29.4 months (95% CI 24.8, 41.2) for high-grade serous carcinoma, 22.4 months (95% CI −,−) for clear cell carcinoma, and 17.8 months (95% CI 3.8, −) for carcinosarcoma.
Table 2.
Multiple regression analysis for overall survival
| All patients (n=96) | Ovarian high-grade serous carcinoma patients (n=62) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | p-value | Hazard Ratio |
95% CI | p- value |
Hazard Ratio |
95% CI | |
| CC-score (referent: CC-0) | CC-1 | <0.0001 | 2.9 | 1.4 – 6.1 | 0.001 | 8.5 | 1.6-45.0 |
| CC-2 | 5.9 | 2.3 – 15.6 | 46.6 | 2.7-811.8 | |||
| CC-3 | 17.2 | 3.6 – 82.6 | 31.2 | 2.5-388.9 | |||
| Pathologic subtype (referent: Mucinous borderline neoplasm) | High-grade serous carcinoma | 0.0014 | 17.8 | 3.9 – 81.8 | NA | ||
| Clear cell carcinoma | 107.8 | 7.7 – 1505.9 | |||||
| Endometrioid carcinoma | 9.9 | 0.8 – 119.9 | |||||
| Granulosa cell tumor | 7.6 | 0.9 – 61.6 | |||||
| Carcinosarcoma | 78.4 | 9.1 – 673.8 | |||||
| Mucinous carcinoma | 3.4 | 0.6 – 20.6 | |||||
| 30-day morbidity (referent: No) | Yes | 0.01 | 2.2 | 1.2 – 4.2 | 0.04 | 3.6 | 0.9-11.2 |
CC-score: completeness of cytoreduction
For the ovarian high-grade serous carcinoma cohort (n=62), the estimated median OS from OPM was 58.3 months (95% CI 50.9 to 78.4 months), with OS probability at 3- and 5-years from OPM of 72.4% (95% CI 59.4% to 81.9%) and 48.8% (95% CI 34.8% to 61.3%), respectively. The estimated median OS from CRS-HIPEC was 26.4 months (95% CI 21.3 to 41.2 months), with OS probability at 3- and 5-years from surgery of 43.1% (95% CI 29.5% to 55.9%) and 23.1% (95% CI 11.5% to 37%), respectively. The estimated median PFS from CRS-HIPEC was 9.6 months (95% CI 7.5 to 13.3 months), with PFS probability at 3- and 5-years from surgery of 11.7% (95% CI 5.2% to 21.3%) and 3.9% (95% CI 0.8% to 11.6%), respectively. Multiple regression analysis for OS in the cohort of patients with ovarian high-grade serous carcinomas demonstrated CC-score (p=0.001), and morbidity at 30 days (p=0.04) to be independent predictors, with no difference in survival related to platinum-sensitivity, treatment of primary or recurrent disease, or administration/response to systemic chemotherapy prior to CRS-HIPEC.(Figures 1–4) By univariate analysis, we found no difference in OS in patients perfused with mitomycin C or cisplatin during HIPEC, however there was a trend towards higher postoperative complications (any grade) in the cisplatin-perfused patients (76.9% vs. 46.9%, p=0.07).
Figure 1.
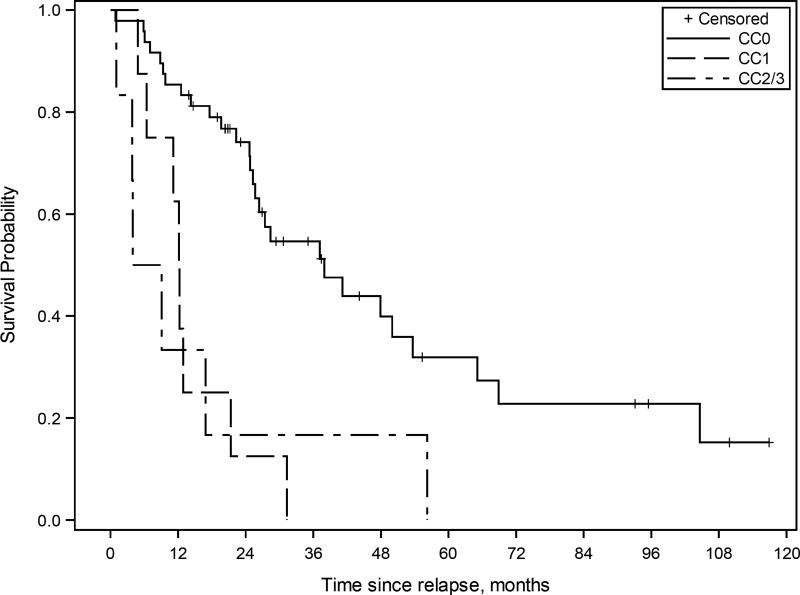
Kaplan-Meier survival curve for patients with ovarian high-grade serous carcinoma treated with multimodality therapy including cytoreductive surgery (CRS)-hyperthermic intraperitoneal chemoperfusion (HIPEC) and systemic chemotherapy (n=62). The estimated median overall survival from CRS-HIPEC was 38 months (95% CI 25.3 to 53.7 months) for CC-0 resection, 12.2 months (95% CI 4.9 to 21.3 months) for CC-1 resection, and 6.5 months (95% CI 1.0 to 56.2 months) for CC-2/3 resection.
Figure 4.
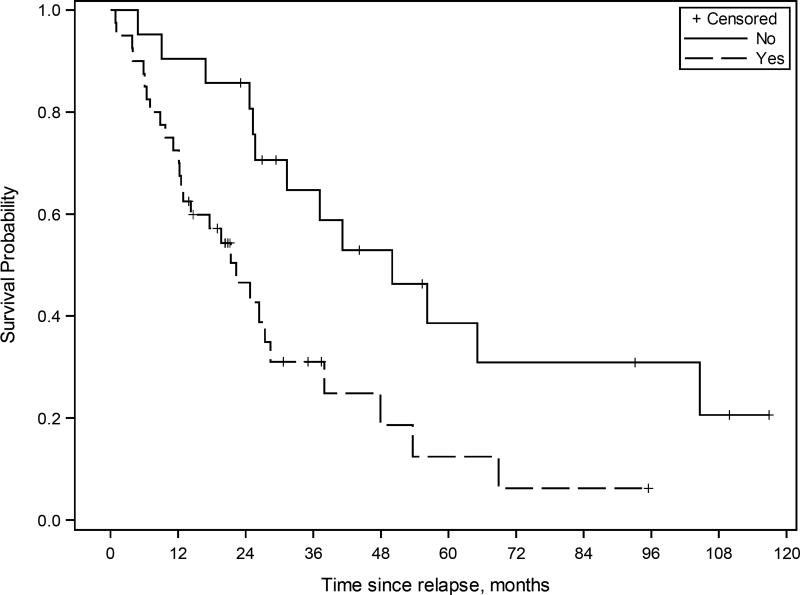
Kaplan-Meier survival curve for patients with ovarian high-grade serous carcinoma treated with multimodality therapy including cytoreductive surgery (CRS)-hyperthermic intraperitoneal chemoperfusion (HIPEC) and systemic chemotherapy (n=62). The estimated median overall survival from CRS-HIPEC was 50 months (95% CI 25.7 to 104.6 months) for patients without 30-day postoperative morbidity, and 22.3 months (95% CI 12.5 to 28.4 months) for patients with 30-day postoperative morbidity.
DISCUSSION
Peritoneal metastasis without systemic dissemination is the major cause of treatment failure and death in ovarian cancer and PPC.(1) This provides a rationale for aggressive locoregional therapies to improve disease control and positively impact long-term survival. Randomized trials and meta-analyses have demonstrated delayed recurrence and improved long-term survival with the addition of IP dwell chemotherapy to standard therapy of CRS and IV chemotherapy for AJCC stage III ovarian cancer.(5–8, 11, 16) However IP dwell chemotherapy has not been widely adopted due to difficulty with administration and increased toxicity.(12) The addition of HIPEC to standard therapy of CRS and IV chemotherapy has been evaluated as an alternative IP treatment strategy in prospective and retrospective studies, with results demonstrating survival advantage regardless of platinum-sensitivity.(27, 29)
Our large single-institution study demonstrates median survival of 38 months (calculated form CRS-HIPEC) and 78 months (calculated from diagnosis of PM) in a heterogeneous group of patients with primary or recurrent OPM and PPC. Our survival data compares less favorably to some published studies of standard therapy (CRS and IV chemotherapy) while it is comparable or better than other series. (4–8, 18–23, 26–28) This disparity appears largely to be a result of differences in disease- and patient-specific inclusion criteria. For example, most of the randomized trials of standard therapy (CRS and IV ± IP chemotherapy) demonstrating median OS ≥ 50 months do not provide information regarding the extent of peritoneal disease burden (PCI or surrogate) and include patients with primary (non-recurrent) disease undergoing optimal CRS. Deraco and colleagues published a prospective phase 2 study of upfront CRS-HIPEC in highly selected OPM patients without prior chemotherapy or resection (similar to patients in clinical trials of standard therapy of CRS and IV chemotherapy) where they demonstrated median survival of > 60 months (61% 5 year survival) and 30 months progression-free survival that is better than most trials of standard therapy.(21) Similar results were published by Fagotti and colleagues for highly selected patients with recurrent OPM undergoing CRS-HIPEC.(27) These data suggest that in highly selected patients, CRS-HIPEC may provide similar or perhaps better survival benefit over standard CRS and IV chemotherapy. On the other hand, Zhang and colleagues performed a pooled analysis of standard therapy (CRS and IV chemotherapy) for recurrent OPM in which 47% of the patients had localized disease recurrence (defined as ≤ 3 lesions) and demonstrated overall survival of 28 months, while survival for patients with multiple areas of recurrence (> 3 lesions) was only 20 months.(25) Our survival data compares favorably to this study (with similar inclusion criteria) suggesting that in patients with extensive peritoneal metastases addition of HIPEC to CRS may provide survival benefit.
Similar to previous publications, residual disease following CRS-HIPEC was an independent predictor of poor survival in this study of patients with OPM and PPC.(9, 25, 34–36) The negative impact of residual tumor nodules has been extensively reported for ovarian cancer and has led to a paradigm shift in surgical approach from “optimal CRS” being defined as < 1 cm residual disease (“minimal residual disease”) after surgery to total macroscopic clearance (“no residual visible disease”).(37) Following an extensive review of published data for over 9000 patients undergoing CRS and IV chemotherapy for advanced ovarian cancer, Bristow and colleagues reported a 5.5% increase in median survival time for each 10% increase in maximal cytoreduction.(9) Similarly, Chi and colleagues compared survival data at their institution in an earlier cohort of patients undergoing less aggressive CRS (54% of patients with residual disease > 1cm) and a later cohort of patients undergoing more aggressive CRS (20% of patients with residual disease > 1 cm) and found improved PFS and OS in the latter cohort.(36) Eisenkop and colleagues prospectively evaluated the impact of and interaction between extent of peritoneal disease and completion of cytoreduction in 408 patients with stage IIIC OPM undergoing CRS followed by IV chemotherapy. While extent of disease and completion of cytoreduction were independent predictors of survival on multivariate analysis, the latter had a much stronger independent effect on survival.(35) du Bois and colleagues performed a meta-analysis of 3120 patients from three prospective randomized trials undergoing CRS and IV chemotherapy for untreated advanced ovarian cancer. Interestingly, they found that the negative impact of incomplete macroscopic resection on survival far outweighed the impact of other biologic prognostic factors like age, performance status, histologic subtype, ascites and stage.(34) More recently, the importance of complete macroscopic resection was also highlighted in patients undergoing secondary CRS for recurrent ovarian cancer.(25) The importance of complete macroscopic resection cannot be over-emphasized in the setting of IP chemotherapy given the limited penetration of locally administered drug up to 3–5 mm.(38) In the randomized trial by Tewari and colleagues studying the benefit of IP dwell chemotherapy, while 5-year survival was no different with and without IP dwell chemotherapy, patients that achieved complete macroscopic resection demonstrated superior 10-year survival (50% vs. 33%).(8) This would suggest that improvement in our ability to eradicate residual disease translates into higher “cure” rates and supports aggressive locoregional therapy. This is also supported by the randomized control trial by Spiliotis et al. in which patients in the HIPEC-group undergoing CC-0 resection had 30.9 months median survival compared to 23.9 months for CC-1 resection and 12.1 months for CC-2 resection.(29) These data support a change in the surgical goals of OPM and PPC from “optimal CRS” (< 1 cm residual disease) to complete macroscopic resection. Once CC-0 resection becomes the standard of care, then the added value of additional therapies can be rigorously assessed.
The extent of peritoneal tumor burden has been shown to influence the ability to achieve complete macroscopic resection.(35) Since the vast majority of advanced ovarian cancers are high-grade serous carcinomas that are highly sensitive to systemic chemotherapy, preoperative systemic chemotherapy has been advocated to optimize resection, especially in patients with high tumor burden.(39, 40) Two randomized control trials demonstrated non-inferiority of short-course neoadjuvant IV chemotherapy prior to CRS compared to up-front surgery with adjuvant IV chemotherapy in patients with stage IIIC and IV OPM and PPC.(41, 42) Conversely, several published observational studies demonstrate worse survival in patients receiving neoadjuvant IV chemotherapy.(43–45) While selection bias may account for this discrepancy (since patients with borderline resectable disease and those with heavier disease burden may be more likely to receive neoadjuvant IV chemotherapy), the potential for selecting chemoresistant clones that are less obvious at surgical exploration may also lead to earlier recurrence and worse survival.(10) Current NCCN guidelines recommend neoadjuvant IV chemotherapy only for patients with high-volume disease who are not surgical candidates. In our study, the use of preoperative IV chemotherapy and response to chemotherapy did not influence survival. In addition, we did not demonstrate significant survival difference between patients with platinum-sensitive versus platinum-resistant/refractory disease, which is in accordance with the larger multi-institutional analysis conducted by Bakrin and colleagues.(46)
Histopathologic subtype was an independent predictor of survival in our study. This is not surprising given that the various subtypes of ovarian cancer have been shown to have distinct predisposing risk factors, genetic aberrations, and altered cellular signaling pathways that influence their response to therapy and oncologic outcomes.(37, 47) Consistent with published data, our study demonstrated better survival for mucinous borderline, low-grade serous carcinoma and endometrioid carcinoma, compared to those with high-grade serous carcinoma, clear cell carcinoma and carcinosarcoma. Finally, postoperative morbidity was an independent predictor of survival in our study and is consistent with prior studies of CRS-HIPEC for a variety of malignancies.(48) This underscores the importance of balancing the potential benefits of aggressive multimodality therapy with the inherent risks associated with CRS-HIPEC. Moreover, our data would suggest against the use of cisplatin perfusion over mitomycin C given the lack of survival benefit and potential increased postoperative morbidity with cisplatin. In our study, Clavien-Dindo grade 3–4 renal complications occurred in three patients receiving cisplatin in the perfusate. These three renal complications occurred earlier in our series when we were using higher doses of cisplatin based on a phase 1 trial of HIPEC with cisplatin conducted by one of our authors while at the NIH (National Institute of Health) that demonstrated a maximum tolerated dose of 250 mg/m2.(49) We serially reduced the cisplatin dose when we encountered renal toxicity at doses of 250, then 225 and 200 mg/m2. We have not encountered any further renal toxicities since we have used a dose of 175 mg/m2, although recent phase I studies of cisplatin have demonstrated maximum tolerated doses of 70–100 mg/m2.(50, 51) Aggressive fluid management is imperative to avoid renal toxicity, therefore in order to mitigate cisplatin-induced renal toxicity, we use a perioperative protocol to flush the kidneys. This includes maintenance of urine output above 200 ml/hour during the operation and for the first 12 hours postoperatively, followed by a urine output of 100 ml/hour for the next 12 hours; as well as administration of sodium thiosulfate bolus and 12 hour continuous infusion that starts prior to cisplatin perfusion. Though mitomycin has not shown significant efficacy via intravenous route in epithelial ovarian cancer, its use in cytoreductive surgery/HIPEC derives from regimens for gastrointestinal malignancies. At our institution, treatment with a platinum-containing agent is preferred for patients with platinum-sensitive EOC; however, it is more toxic than mitomycin C, particularly in regards to renal dysfunction. In patients who are known to be platinum-resistant or in whom performance status, medical comorbidities, or end-organ disease prohibit the use of a platinum-containing agent, mitomycin C is preferred. Our institution has previously shown success using mitomycin C for epithelial ovarian cancer patients.
Limitations of our study include the relatively small sample size, heterogeneity of the patient population and inherent bias associated with the retrospective nature of the data. There is also some missing data, especially for analysis regarding use of and response to preoperative IV chemotherapy, as well as platinum-sensitivity that may have biased the results. Finally, we can only infer any potential benefit from the addition of HIPEC to multimodality therapy since direct comparison to standard therapy (CRS + IV chemotherapy ± IP chemotherapy) was not performed.
CONCLUSIONS
In conclusion, our data demonstrate promising survival results in patients with primary or recurrent OPM and PPC undergoing CRS-HIPEC. The addition of HIPEC to standard multimodality therapy allows for regional delivery of chemotherapy, while overcoming some of the logistical issues and complications associated with IP dwell chemotherapy that have prevented its widespread use. Results from ongoing randomized trials of CRS-HIPEC for primary and recurrent OPM and PPC will clarify the role of this aggressive locoregional approach.
Figure 2.
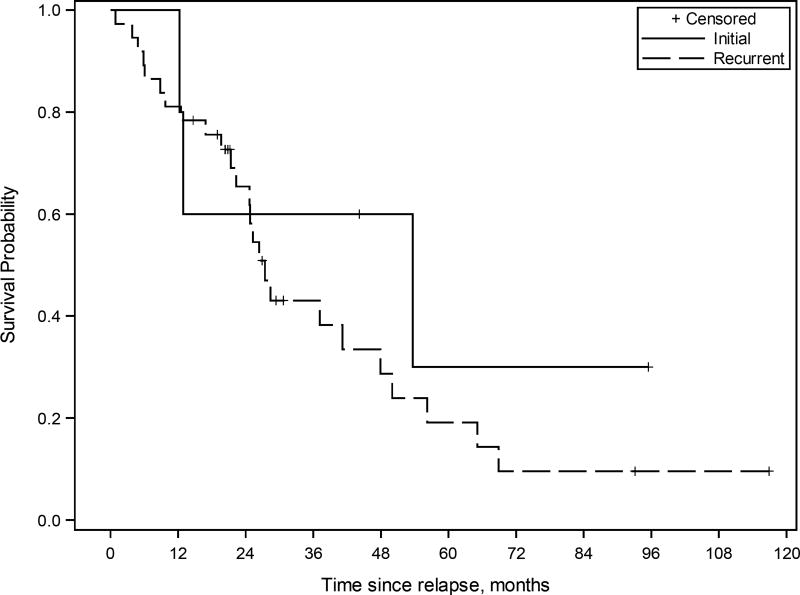
Kaplan-Meier survival curve for patients with ovarian high-grade serous carcinoma treated with multimodality therapy including cytoreductive surgery (CRS)-hyperthermic intraperitoneal chemoperfusion (HIPEC) and systemic chemotherapy (n=62). The estimated median overall survival from CRS-HIPEC was 27.4 months (95% CI 22.3 to 47.9 months) for treatment of recurrent disease, and 53.7 months (95% CI 12.2 to - months) for initial treatment of the disease.
Figure 3.
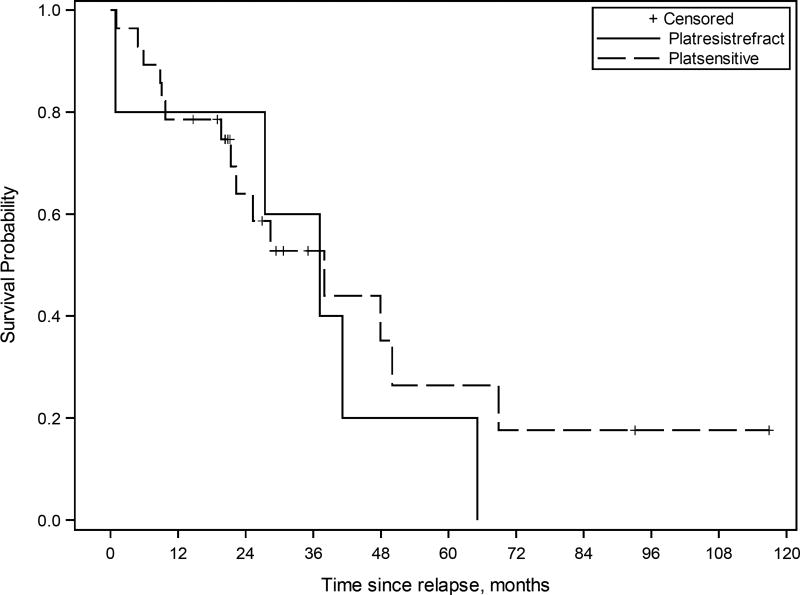
Kaplan-Meier survival curve for patients with ovarian high-grade serous carcinoma treated with multimodality therapy including cytoreductive surgery (CRS)-hyperthermic intraperitoneal chemoperfusion (HIPEC) and systemic chemotherapy (n=62). The estimated median overall survival from CRS-HIPEC was 38.0 months (95% CI 21.3 to 68.9 months) for platinum-sensitive disease (“platsensitive”), and 37.2 months (95% CI 0.9 to 65.1 months) for platinum-resistant or refractory disease (“platresistrefract”).
SYNOPSIS.
Cytoreductive surgery with hyperthermic intraperitoneal chemoperfusion, as a component of multimodality therapy, provides meaningful survival in patients with primary or recurrent ovarian peritoneal metastases and primary peritoneal cancer, especially in patients experiencing complete macroscopic resection without postoperative complications.
Acknowledgments
This work was also partially funded by generous support from Valarie Koch and the New Era Cap Company. The project was supported by the National Institutes of Health through Grant Number UL1TR000005, using a Red cap maintained database.
Footnotes
Disclosures: None
References
- 1.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374(9698):1371–82. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351(24):2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Amate P, Huchon C, Dessapt AL, Bensaid C, Medioni J, Le Frere Belda MA, et al. Ovarian cancer: sites of recurrence. Int J Gynecol Cancer. 2013;23(9):1590–6. doi: 10.1097/IGC.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 7.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(4):1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 8.Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33(13):1460–6. doi: 10.1200/JCO.2014.55.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 10.Narod S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol. 2016;13(4):255–61. doi: 10.1038/nrclinonc.2015.224. [DOI] [PubMed] [Google Scholar]
- 11.Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev. 2006;1:CD005340. doi: 10.1002/14651858.CD005340.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Rowan K. Intraperitoneal therapy for ovarian cancer: why has it not become standard? J Natl Cancer Inst. 2009;101(11):775–7. doi: 10.1093/jnci/djp151. [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7(1):69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 14.Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24(24):4011–9. doi: 10.1200/JCO.2006.07.1142. [DOI] [PubMed] [Google Scholar]
- 15.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27(36):6237–42. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 16.Markman M. Intraperitoneal chemotherapy in the management of malignant disease. Expert Rev Anticancer Ther. 2001;1(1):142–8. doi: 10.1586/14737140.1.1.142. [DOI] [PubMed] [Google Scholar]
- 17.Roviello F, Caruso S, Marrelli D, Pedrazzani C, Neri A, De Stefano A, et al. Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: state of the art and future developments. Surg Oncol. 2011;20(1):e38–54. doi: 10.1016/j.suronc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Bakrin N, Classe JM, Pomel C, Gouy S, Chene G, Glehen O. Hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. J Visc Surg. 2014;151(5):347–53. doi: 10.1016/j.jviscsurg.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Chiva LM, Gonzalez-Martin A. A critical appraisal of hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of advanced and recurrent ovarian cancer. Gynecol Oncol. 2015;136(1):130–5. doi: 10.1016/j.ygyno.2014.11.072. [DOI] [PubMed] [Google Scholar]
- 20.Coccolini F, Campanati L, Catena F, Ceni V, Ceresoli M, Jimenez Cruz J, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol. 2015;26(1):54–61. doi: 10.3802/jgo.2015.26.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deraco M, Kusamura S, Virzi S, Puccio F, Macri A, Famulari C, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: multi-institutional phase-II trial. Gynecol Oncol. 2011;122(2):215–20. doi: 10.1016/j.ygyno.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Helm CW, Richard SD, Pan J, Bartlett D, Goodman MD, Hoefer R, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer: first report of the HYPER-O registry. Int J Gynecol Cancer. 2010;20(1):61–9. doi: 10.1111/IGC.0b013e3181c50cde. [DOI] [PubMed] [Google Scholar]
- 23.Mulier S, Claes JP, Dierieck V, Amiel JO, Pahaut JP, Marcelis L, et al. Survival benefit of adding Hyperthermic IntraPEritoneal Chemotherapy (HIPEC) at the different time-points of treatment of ovarian cancer: review of evidence. Curr Pharm Des. 2012;18(25):3793–803. doi: 10.2174/138161212802002616. [DOI] [PubMed] [Google Scholar]
- 24.Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol. 2009;112(1):265–74. doi: 10.1016/j.ygyno.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Zang RY, Harter P, Chi DS, Sehouli J, Jiang R, Trope CG, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105(7):890–6. doi: 10.1038/bjc.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boisen MM, Richard SD, Holtzman MP, Edwards RP, Kelley JL, Choudry MH, et al. Hyperthermic intraperitoneal chemotherapy for epithelial ovarian cancers: is there a role? J Gastrointest Oncol. 2016;7(1):10–7. doi: 10.3978/j.issn.2078-6891.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fagotti A, Costantini B, Petrillo M, Vizzielli G, Fanfani F, Margariti PA, et al. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: a case-control study on survival in patients with two year follow-up. Gynecol Oncol. 2012;127(3):502–5. doi: 10.1016/j.ygyno.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Fagotti A, Costantini B, Vizzielli G, Perelli F, Ercoli A, Gallotta V, et al. HIPEC in recurrent ovarian cancer patients: morbidity-related treatment and long-term analysis of clinical outcome. Gynecol Oncol. 2011;122(2):221–5. doi: 10.1016/j.ygyno.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22(5):1570–5. doi: 10.1245/s10434-014-4157-9. [DOI] [PubMed] [Google Scholar]
- 30.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 31.Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J. 2009;15(3):204–11. doi: 10.1097/PPO.0b013e3181a9c6f0. [DOI] [PubMed] [Google Scholar]
- 32.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 33.Friedlander M, Trimble E, Tinker A, Alberts D, Avall-Lundqvist E, Brady M, et al. Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21(4):771–5. doi: 10.1097/IGC.0b013e31821bb8aa. [DOI] [PubMed] [Google Scholar]
- 34.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115(6):1234–44. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 35.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90(2):390–6. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 36.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114(1):26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Moreno S, Gonzalez-Bayon LA, Ortega-Perez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol. 2010;2(2):68–75. doi: 10.4251/wjgo.v2.i2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009;16(8):2315–20. doi: 10.1245/s10434-009-0558-6. [DOI] [PubMed] [Google Scholar]
- 40.Vergote I, Amant F, Leunen K. Neoadjuvant chemotherapy in advanced ovarian cancer: what kind of evidence is needed to convince US gynaecological oncologists? Gynecol Oncol. 2010;119(1):1–2. doi: 10.1016/j.ygyno.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 42.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–57. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 43.Colombo PE, Labaki M, Fabbro M, Bertrand M, Mourregot A, Gutowski M, et al. Impact of neoadjuvant chemotherapy cycles prior to interval surgery in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2014;135(2):223–30. doi: 10.1016/j.ygyno.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Rauh-Hain JA, Nitschmann CC, Worley MJ, Jr, Bradford LS, Berkowitz RS, Schorge JO, et al. Platinum resistance after neoadjuvant chemotherapy compared to primary surgery in patients with advanced epithelial ovarian carcinoma. Gynecol Oncol. 2013;129(1):63–8. doi: 10.1016/j.ygyno.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Rosen B, Laframboise S, Ferguson S, Dodge J, Bernardini M, Murphy J, et al. The impacts of neoadjuvant chemotherapy and of debulking surgery on survival from advanced ovarian cancer. Gynecol Oncol. 2014;134(3):462–7. doi: 10.1016/j.ygyno.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Ejso-Eur J Surg Onc. 2013;39(12):1435–43. doi: 10.1016/j.ejso.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 47.Lalwani N, Prasad SR, Vikram R, Shanbhogue AK, Huettner PC, Fasih N. Histologic, molecular, and cytogenetic features of ovarian cancers: implications for diagnosis and treatment. Radiographics. 2011;31(3):625–46. doi: 10.1148/rg.313105066. [DOI] [PubMed] [Google Scholar]
- 48.Haslinger M, Francescutti V, Attwood K, McCart JA, Fakih M, Kane JM, 3rd, et al. A contemporary analysis of morbidity and outcomes in cytoreduction/hyperthermic intraperitoneal chemoperfusion. Cancer Med. 2013;2(3):334–42. doi: 10.1002/cam4.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartlett DL, Buell JF, Libutti SK, Reed E, Lee KB, Figg WD, et al. A phase I trial of continuous hyperthermic peritoneal perfusion with tumor necrosis factor and cisplatin in the treatment of peritoneal carcinomatosis. Cancer. 1998;83(6):1251–61. [PubMed] [Google Scholar]
- 50.Gouy S, Ferron G, Glehen O, Bayar A, Marchal F, Pomel C, et al. Results of a multicenter phase I dose-finding trial of hyperthermic intraperitoneal cisplatin after neoadjuvant chemotherapy and complete cytoreductive surgery and followed by maintenance bevacizumab in initially unresectable ovarian cancer. Gynecol Oncol. 2016;142(2):237–42. doi: 10.1016/j.ygyno.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 51.Zivanovic O, Abramian A, Kullmann M, Fuhrmann C, Coch C, Hoeller T, et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer. 2015;136(3):699–708. doi: 10.1002/ijc.29011. [DOI] [PubMed] [Google Scholar]