Abstract
Purpose
Chromosomal polymorphisms are associated with infertility, but their effects on assisted reproductive outcomes are still quite conflicting, especially after IVF treatment. This study evaluated the role of chromosomal polymorphisms of different genders in IVF pregnancy outcomes.
Methods
Four hundred and twenty-five infertile couples undergoing IVF treatment were divided into three groups: 214 couples with normal chromosomes (group A, control group), 86 couples with female polymorphisms (group B), and 125 couples with male polymorphisms (group C). The pregnancy outcomes after the first and cumulative transfer cycles were analyzed, and the main outcome measures were live birth rate (LBR) after the first transfer cycle and cumulative LBR after a complete IVF cycle.
Results
Comparison of pregnancy outcomes after the first transfer cycle within group A, group B, and group C demonstrated a similar LBR as well as other rates of implantation, clinical pregnancy, early miscarriage, and ongoing pregnancy (P > 0.05). However, the analysis of cumulative pregnancy outcomes indicated that compared with group A, group C had a significantly lower LBR per cycle (80.4 vs 68.00%), for a rate ratio of 1.182 (95% CI 1.030 to 1.356, P = 0.01) and a significantly higher cumulative early miscarriage rate (EMR) among clinical pregnancies (7.2 vs 14.7%), for a rate ratio of 0.489 (95% CI 0.248 to 0.963, P = 0.035).
Conclusion
Couples with chromosomal polymorphisms in only male partners have poor pregnancy outcomes after IVF treatment manifesting as high cumulative EMR and low LBR after a complete cycle.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0951-1) contains supplementary material, which is available to authorized users.
Keywords: Chromosomal polymorphisms, IVF, Cumulative live birth rate, Miscarriage
Introduction
Chromosomal polymorphisms, also called chromosomal variations or heteromorphisms, refer to variations of size and staining in heterochromatic regions of the genome [1]. They are tandemly organized and highly repeated noncoding sequences of DNA and usually manifest as elongation or contraction of the heterochromatin on the long arm of chromosome 1, 9, 16, Y, as well as the short arms, satellites, and stalks of the acrocentric D and G group chromosomes (13, 14, 15, 21, and 22) [2]. Chromosomal polymorphisms are more than “harmless,” and it was reported that couples with chromosomal polymorphisms have a higher risk of reproductive failure, spontaneous miscarriage, and bad obstetric histories [2–10].
However, the conclusions drawn from different studies on the association between polymorphisms and assisted reproductive outcomes are quite inconsistent. Hong Y et al. compared the pregnancy outcomes of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) between 82 couples with chromosomal polymorphisms in only females, 187 couples with chromosomal polymorphisms in only males, and 1402 controls and found no significant differences; only the early miscarriage rate of male carriers tended to be higher [11]; while Guo T et al. observed lower fertilization rate and clinical pregnancy rate in male carriers with severe oligozoospermia under ICSI treatment compared with non-carriers [12]; and Xu X concluded that both male and female polymorphisms have adverse effects on the pregnancy outcomes after fresh IVF/ICSI cycle [13].
The conclusions of above studies were exclusively based on the analysis of assisted reproductive outcomes after the first transfer cycle. The live birth rate (LBR), especially LBR per woman or per cycle following a single oocyte retrieval, is the most concerned issue for clinicians and patients, but never analyzed as a main outcome measure in previous chromosomal polymorphism studies.
Besides, chromosomal polymorphisms tend to occur more frequently in male partners than female partners within infertile and recurrent spontaneous abortion (RSA) couples [2, 5, 10, 14], and their effect on spermatogenesis has been proposed by Yakin K et al. and Guo T et al. [12, 15]; thus, the emphasis of previous studies is more often placed on ICSI treatment instead of IVF.
Therefore, this retrospective study is to comprehensively analyze the pregnancy outcomes after the first and cumulative transfer cycles in 425 infertile couples undergoing IVF treatment in our department from 2013 to 2014 and evaluate the role of chromosomal polymorphisms of different genders in the IVF pregnancy outcomes.
Materials and methods
Subjects
From January 2013 to October 2014, a total of 425 infertile couples undergoing IVF treatment at Reproductive Medical Center of Shandong University (SDU reproductive center) were recruited in this retrospective case-control study. Data from the first and cumulative embryo transfer cycles were analyzed separately, and the latter means all transfer cycles following a single oocyte retrieval. Female age was from 20 to 40 years old, and basal follicle-stimulating hormone (FSH) was under 15 IU/L.
The exclusion criteria included female partners with the following abnormalities: (1) abnormal chromosome karyotypes, not including inv(9); (2) anatomical defects of reproductive system diagnosed under ultrasonography, hysterosalpingography, and hysteroscopy; (3) histories of fertilization failure, recurrent implantation failure, and RSA; (4) endocrinal, infectious, and immunological diseases, such as premature ovarian insufficiency, abnormality of thyroid function, infection of Rubella virus, cytomegalovirus, and other abnormalities having apparent impact on pregnancy outcomes.
The infertile couples were stratified by presence or absence of a polymorphic chromosome for different genders. Group A (control group) consisted of 214 couples both with normal chromosomes, group B consisted of 86 couples with polymorphisms in only female partners, while group C consisted of 125 couples with polymorphisms in only male partners.
Ethical approval
All procedures performed in our study involving human participants were in accordance with the ethical standards of the institutional review board of reproductive medicine and with the 1964 Helsinki Declaration and its later amendments. For this type of study, formal consent is not required. Informed consent was obtained from all individual participants included in the study.
Karyotyping
Karyotype analysis was carried out for all infertile couples before IVF treatment. Metaphase chromosomes from peripheral lymphocytes were cultured for 72 h and stained with G-banding techniques under 450-band resolutions. An analysis of at least 30 metaphases per patient was needed. If necessary, C-banding and R-banding staining methods were adopted to assist karyotype analysis. Chromosomal polymorphisms were reported according to the International System for Chromosome Nomenclature 2009.
Ovarian stimulation and oocyte retrieval
All female partners underwent controlled ovarian stimulation with GnRH agonist (Decapeptyl, Ferring, Guangdong, China) combined with recombinant FSH (Gonal-F, Merck Serono, Geneva, Switzerland) according to the long or short protocol on the basis of ovarian reserve function. The long protocol started with GnRH agonist administrated in the mid luteal phase of the previous cycle and combined with recombinant FSH when satisfactory pituitary desensitization was achieved in this cycle. And the short protocol started with the administration of GnRH agonist and recombinant FSH together on day 2 or 3 of the cycle. Then, the dosage of recombinant FSH was regulated in the light of follicle growth and serum E2 concentration. And oocytes were retrieved through transvaginally ultrasound-guided puncture at 36 h after hCG (5000–10,000 IU, im; pregnyl, Livzon, Guangdong, China) administration when there were at least two follicles with diameter beyond 18 mm.
Embryo transfer and pregnancy confirmation
In general, one or two fresh embryos were transferred on day 2 or 3 after fertilization if permitting. And frozen-thawed embryos transfer was chosen instead if OHSS, high progesterone occurred. Luteal phase support was carried out by means of injecting natural progesterone in oil (progesterone injection, General, Shanghai, China) or taking orally dydrogestrone (Duphaston, Abbott Health Care Products B.V.) throughout the whole first-trimester pregnancy. Biochemical pregnancy was confirmed 2 weeks after embryo transfer by rise of β-human chorionic gonadotropin (β-HCG) in serum (over 10 IU/L). And clinical pregnancy was confirmed 35 days after embryo transfer when an intrauterine gestational sac was detected with ultrasound examination. Follow-ups were implemented at fixed time until termination of the cycle following a single oocyte retrieval (live birth, no available embryos, or abandonment).
Outcome measures
Main outcome measures were LBR after the first transfer cycle (LBR per transfer cycle) and cumulative LBR after a complete IVF cycle (LBR per cycle). A complete cycle is defined as all transfer cycles in an IVF cycle started with oocyte stimulation, ended with live birth or no available embryos or abandonment.
Other outcome measures include implantation rate (IR), biochemical pregnancy rate (BPR), biochemical pregnancy abortion rate (BPAR), clinical pregnancy rate (CPR), early miscarriage rate (EMR), ongoing pregnancy rate (OPR), and preterm birth rate (PBR) in the first and cumulative transfer cycles.
EMR means the number of transfer cycles with miscarriage during the first-trimester pregnancy among the transfer cycles confirmed with clinical pregnancy. And OPR means the number of transfer cycles with pregnancy beyond 20 gestational weeks among all transfer cycles.
Statistical analysis
Continuous variables were described as mean ± standard deviation, while categorical variables as frequency and percentage. Differences were examined with independent sample t test or Pearson chi-square test through the IBM SPSS Statistics 19 program for Windows (Armonk, NY, USA). And p < 0.05 was considered to be statistically significant.
A binary logistic regression model was also conducted to examine the impact of chromosomal polymorphisms of different genders on pregnancy outcomes and adjust potential cofounders. Chromosomal polymorphisms, long protocol or short protocol, embryos transfer (ET), or cryopreserved embryos transfer (CET) was referred to categorical variables, and chromosomal polymorphisms of different genders as multicategorical variables were transformed to dummy variables.
Besides, a Kaplan-Meier method was applied to further compare cumulative LBR and analyze the cumulative effect of chromosomal polymorphisms.
Results
Basic characteristics
The basic characteristics of group A (couples both with normal chromosomes), group B (couples with polymorphisms in only female partners), and group C (couples with polymorphisms in only male partners) are listed in Table 1. Basic characteristics of group B and group C were compared with group A separately, and no statistically significant differences were observed regarding female age, female body mass index (BMI), female AMH, female basal FSH, female basal PRL, female basal TSH, antral follicle count (AFC), E2 on HCG day, and sperm progressive motility (P > 0.05). Nevertheless, the number of oocytes retrieved and high-quality embryos on day 3 in group B was significantly less than group A (P = 0.017 and P = 0.020, respectively). And sperm concentration in group C was significantly lower than group A (49.71 ± 12.53 × 106⁄mL and 52.56 ± 12.79 × 106⁄mL, respectively, P = 0.047).
Table 1.
Comparison of basic characteristics in infertile couples
| Group A (min, max) | Group B (min, max) | P1 | Group C (min, max) | P2 | |
|---|---|---|---|---|---|
| Female age | 30.06 ± 3.93 (23, 40) |
30.64 ± 4.66 (20, 40) |
0.276 | 30.86 ± 4.40 (22, 40) |
0.087 |
| BMI | 23.45 ± 3.42 (16.73, 35.8) |
23.45 ± 4.02 (15.81, 38.77) |
0.987 | 23.15 ± 3.29 (17.22, 32.42) |
0.429 |
| AMH (ng/mL) | 2.60 ± 2.15 (0.090, 16.042) |
2.54 ± 2.26 (0.079, 11.099) |
0.817 | 2.94 ± 2.49 (0.079, 14.595) |
0.194 |
| FSH (IU/L) | 6.66 ± 1.74 (3.28, 14.12) |
6.72 ± 1.86 (3.20, 14.71) |
0.778 | 6.91 ± 1.77 (3.77, 14.73) |
0.210 |
| PRL (ng/mL) | 15.45 ± 6.04 (4.10, 34.29) |
15.02 ± 5.27 (7.09, 30.91) |
0.565 | 15.88 ± 5.73 (5.06, 33.10) |
0.520 |
| TSH (μIU/mL) | 2.41 ± 1.15 (0.370, 5.75) |
2.50 ± 1.76 (0.354, 15.840) |
0.587 | 2.32 ± 1.03 (0.703, 5.940) |
0.477 |
| AFC | 16.30 ± 7.10 (3, 68) |
15.00 ± 5.99 (2, 33) |
0.135 | 15.90 ± 5.57 (6, 39) |
0.590 |
| Sperm concentration (×106⁄mL) | 52.56 ± 12.79 (20, 90) |
49.48 ± 12.42 (24, 75) |
0.058 | 49.71 ± 12.53 (5, 80) |
0.047 |
| Sperm progressive motility (%) | 40.22 ± 9.42 (5, 64) |
38.73 ± 8.90 (13, 62) |
0.210 | 40.57 ± 9.11 (13, 65) |
0.740 |
| E2 level on HCG day (pg/mL) | 3768.69 ± 1944.45 (759, 9973) |
3946.35 ± 4688.86 (656.7, 43,681) |
0.643 | 3944.79 ± 2136.00 (1147, 10,310) |
0.439 |
| No. of oocytes retrieved | 12.02 ± 5.17 (1, 28) |
10.42 ± 5.28 (2, 30) |
0.017 | 12.02 ± 5.80 (1, 33) |
0.993 |
| No. of high-quality embryos on D3 | 4.43 ± 2.93 (0, 13) |
3.55 ± 3.089 (0, 13) |
0.020 | 3.89 ± 3.01 (0, 13) |
0.102 |
Plus–minus values are means ± SD
Group A couples with normal chromosomes, group B couples with polymorphisms in only female partners, group C couples with polymorphisms in only male partners, BMI female body mass index, AFC antral follicle count, P1 comparison of group B with group A, P2 comparison of group C with group A
p < 0.05 was considered to be statistically significant
Pregnancy outcomes after the first transfer cycle
Comparison of pregnancy outcomes after the first transfer cycle within group A, group B, and group C is shown in Table 2. No significant difference was found in LBR as well as IR, BPR, BPAR, CPR, EMR, OPR, and PBR among these three groups (P > 0.05).
Table 2.
Comparison of pregnancy outcomes in the first transfer cycles
| Group A | Group B | Rate ratio | P1 | Group C | Rate ratio | P2 | |
|---|---|---|---|---|---|---|---|
| ETC = 214 TE = 403 |
ETC = 86 TE = 160 |
(95% CI) | ETC = 125 TE = 225 |
(95% CI) | |||
| IR% | 46.15 (186/403) |
43.13 (69/160) |
1.070 (0.870–1.316) |
0.515 | 47.11 (106/225) |
0.980 (0.823–1.166) |
0.818 |
| BPR% | 68.22 (146/214) |
62.79 (54/86) |
1.087 (0.902–1.309) |
0.367 | 65.60 (82/125) |
1.040 (0.889–1.216) |
0.619 |
| BPAR% | 4.10 (6/146) |
9.26 (5/54) |
0.444 (0.141–1.395) |
0.285 | 4.88 (4/82) |
0.842 (0.245–2.899) |
1.0 |
| CPR% | 63.55 (136/214) |
55.81 (48/86) |
1.139 (0.920–1.410) |
0.213 | 62.40 (78/125) |
1.018 (0.859–1.207) |
0.832 |
| EMR% | 9.56 (13/136) |
10.42 (5/48) |
0.918 (0.345–2.439) |
1.00 | 11.54 (9/78) |
0.828 (0.371–1.849) |
0.646 |
| OPR% | 56.07 (120/214) |
50.00 (43/86) |
1.121 (0.880–1.429) |
0.339 | 52.80 (66/125) |
1.062 (0.866–1.302) |
0.559 |
| PBR% | 10.83 (13/120) |
13.95 (6/43) |
0.776 (0.315–1.914) |
0.584 | 18.18 (12/66) |
0.596 (0.289–1.230) |
0.160 |
| LBR% | 54.67 (117/214) |
50.00 (43/86) |
1.093 (0.857–1.396) |
0.463 | 52.00 (65/125) |
1.051 (0.854–1.294) |
0.634 |
Statistical method: chi-square test
ETC embryo transfer cycle, TE transferred embryos, IR implantation rate, BPR biochemical pregnancy rate, BPAR biochemical pregnancy abortion rate, CPR clinical pregnancy rate, EMR early miscarriage rate, OPR ongoing pregnancy rate, PBR preterm birth rate, LBR live birth rate, P1 comparison of group B with group A, P2 comparison of group C with group A
p < 0.05 was considered to be statistically significant
A binary logistic regression model was conducted to comprehensively evaluate the impact of chromosomal polymorphisms, female age, female BMI, ovarian reserve function, female baseline hormone level, semen quality, ovarian stimulation protocols, and outcomes of ovarian stimulation on pregnancy outcomes. As shown in Supplemental Table 1, on basis of ORs yielded by the model, the use of long protocol tends to produce a better CPR and LBR (OR for CPR 2.327, 95% confidence interval [CI] 1.323 to 4.094, P = 0.003; OR for LBR 2.107, 95% CI 1.211 to 3.667, P = 0.008). For EMR, the transfer of high-quality embryos can reduce the risk of early miscarriage (OR 0.825, 95% CI 0.689 to 0.988, P = 0.037); and TSH within normal range displayed a negative correlation with EMR (OR 0.548, 95% CI 0.337 to 0.891, P = 0.015). All other relative variables demonstrated no or a fairly weak effect on CPR, EMR, and LBR. Adjusting for these potential cofounders, couples with one carrier of chromosomal polymorphisms presented a comparable CPR, EMR, and LBR with those non-carriers.
Pregnancy outcomes after cumulative transfer cycles
Cumulative pregnancy outcomes in IVF treatment were analyzed and compared within three groups, as shown Table 3. In comparison with group A, group C demonstrated a significantly lower cumulative LBR per cycle (LBR-c) (80.4 vs 68.00%), for a rate ratio of 1.182 (95% CI 1.030 to 1.356, P = 0.01) and a significantly higher cumulative EMR among clinical pregnancies (7.2 vs 14.7%), for a rate ratio of 0.489 (95% CI 0.248 to 0.963, P = 0.035); and group B showed a significantly higher cumulative BPAR among biochemical pregnancies (4.8 vs 12.8%), for a rate ratio of 0.375 (95% CI 0.137 to 0.861, P = 0.018). It is worth mentioning that compared to the control group, LBR-c of group B was significantly lower (80.4 vs 69.8%, P = 0.047), but the rate ratio of 1.152 (95% CI 0.988 to 1.344) denied its correlation with female polymorphisms. All other cumulative pregnancy outcomes were comparable within three groups.
Table 3.
Comparison of pregnancy outcomes in the cumulative transfer cycles
| Group A | Group B | Rate ratio | P1 | Group C | Rate ratio | P2 | |
|---|---|---|---|---|---|---|---|
| Women = 214 ETC = 311 TE = 536 |
Women = 86 ETC = 116 TE = 198 |
(95% CI) | Women = 125 ETC = 173 TE = 295 |
(95% CI) | |||
| IR% | 48.5 (260/536) |
46.5 (92/198) |
1.044 (0.878–1.241) |
0.623 | 47.5 (140/295) |
1.022 (0.881–1.186) |
0.772 |
| BPR% | 66.9 (208/311) |
67.2 (78/116) |
0.995 (0.857–1.155) |
0.944 | 67.1 (116/173) |
0.997 (0.875–1.136) |
0.969 |
| BPAR% | 4.8 (10/208) |
12.8 (10/78) |
0.375 (0.137–0.861) |
0.018 | 6.0 (7/116) |
0.797 (0.312–2.037) |
0.635 |
| CPR% | 62.7 (195/311) |
58.6 (68/116) |
1.070 (0.898–1.275) |
0.441 | 63.0 (109/173) |
0.995 (0.863–1.148) |
0.947 |
| EMR% | 7.2 (14/195) |
10.3 (7/68) |
0.697 (0.294–1.655) |
0.415 | 14.7 (16/109) |
0.489 (0.248–0.963) |
0.035 |
| OPR% | 57.2 (178/311) |
52.6 (61/116) |
1.088 (0.893–1.326) |
0.389 | 51.4 (89/173) |
1.113 (0.935–1.424) |
0.220 |
| PBR% | 12.6 (22/175) |
10.0 (6/60) |
1.257 (0.535–2.952) |
0.596 | 15.1 (13/86) |
0.832 (0.441–1.570) |
0.571 |
| LBR-t% | 55.3 (172/311) |
51.7 (60/116) |
1.069 (0.873–1.309) |
0.509 | 49.1 (85/173) |
1.126 (0.939–1.350) |
0.192 |
| LBR-c% | 80.4 (172/214) |
69.8 (60/86) |
1.152 (0.988–1.344) |
0.047 | 68.0 (85/125) |
1.182 (1.030–1.356) |
0.010 |
Statistical method: chi-square test
ETC embryo transfer cycle, TE transferred embryos, IR implantation rate, BPR biochemical pregnancy rate, BPAR biochemical pregnancy abortion rate, CPR clinical pregnancy rate, EMR early miscarriage rate, OPR ongoing pregnancy rate, PBR preterm birth rate, LBR-t live birth rate per transfer cycle, LBR-c live birth rate per cycle, P1 comparison of group B with group A, P2 comparison of group C with group A
p < 0.05 was considered to be statistically significant
A binary logistic regression model was also conducted to testify the impact of chromosomal polymorphisms on cumulative pregnancy outcomes and adjust the potential cofounders. The results turned out as shown in Table 4, on basis of ORs yielded by the model, male polymorphisms can increase the risk of miscarriage and exerted a negative effect on cumulative live births after a complete IVF cycle (OR for EMR 2.525, 95% CI 1.080 to 5.906, P = 0.033; OR for LBR-c 0.552, 95% CI 0.319 to 0.955, P = 0.034). And the long stimulation protocol was proved to have a positive impact on cumulative CPR (OR 2.575, 95% CI 1.283 to 5.167, P = 0.008) and LBR-c (OR 1.977, 95% CI 1.067 to 3.660, P = 0.03), while TSH within normal range displayed a negative correlation with cumulative EMR (OR 0.612, 95% CI 0.404 to 0.927, P = 0.021). All other relative variables demonstrated no or a fairly weak effect on cumulative CPR, EMR, and LBR.
Table 4.
Estimated OR for CPR, early miscarriage rate, and LBR using the binary logistic regression model in the cumulative transfer cycle
| CPR | EMR | LBR-c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | |
| P of Fa | 0.238 | 0.656 | 0.325–1.322 | 0.533 | 1.403 | 0.484–4.073 | 0.412 | 0.767 | 0.408–1.444 |
| P of Ma | 0.632 | 0.850 | 0.437–1.654 | 0.033 | 2.525 | 1.080–5.906 | 0.034 | 0.552 | 0.319–0.955 |
| Female age | 0.001 | 0.882 | 0.821–0.948 | 0.921 | 1.005 | 0.911–1.109 | 0.020 | 0.931 | 0.876–0.989 |
| BMI | 0.123 | 1.075 | 0.981–1.179 | 0.283 | 1.060 | 0.953–1.180 | 0.270 | 0.961 | 0.895–1.032 |
| AMH | 0.481 | 0.946 | 0.810–1.104 | 0.390 | 0.915 | 0.747–1.120 | 0.334 | 0.940 | 0.829–1.066 |
| FSH | 0.289 | 1.095 | 0.926–1.296 | 0.118 | 0.799 | 0.603–1.059 | 0.759 | 1.023 | 0.885–1.182 |
| PRL | 0.492 | 0.983 | 0.937–1.032 | 0.202 | 1.041 | 0.978–1.109 | 0.403 | 0.983 | 0.943–1.024 |
| TSH | 0.070 | 0.794 | 0.619–1.019 | 0.021 | 0.612 | 0.404–0.927 | 0.112 | 0.844 | 0.684–1.040 |
| AFC | 0.270 | 0.972 | 0.924–1.022 | 0.703 | 1.015 | 0.941–1.095 | 0.977 | 1.001 | 0.956–1.048 |
| Sperm concentration | 0.748 | 0.996 | 0.973–1.020 | 0.946 | 1.001 | 0.968–1.035 | 0.950 | 0.999 | 0.979–1.020 |
| Sperm progressive motility | 0.968 | 0.999 | 0.968–1.032 | 0.441 | 1.019 | 0.972–1.067 | 0.717 | 0.995 | 0.967–1.023 |
| Long or short protocol | 0.008 | 2.575 | 1.283–5.167 | 0.917 | 0.946 | 0.332–2.696 | 0.030 | 1.977 | 1.067–3.660 |
| E2 on HCG day | 0.396 | 1.000 | 1.000–1.000 | 0.044 | 1.000 | 0.999–1.000 | 0.497 | 1.000 | 1.000–1.000 |
| No. of oocytes obtained | 0.119 | 1.072 | 0.982–1.170 | 0.182 | 1.083 | 0.963–1.218 | 0.394 | 1.031 | 0.961–1.106 |
| No. of high-quality D3 embryos t | 0.001 | 1.256 | 1.095–1.440 | 0.440 | 0.942 | 0.808–1.097 | 0.002 | 1.189 | 1.068–1.324 |
| ET/CET | 0.925 | 1.053 | 0.358–3.098 | 0.183 | 2.234 | 0.684–7.291 | 0.623 | 1.263 | 0.498–3.208 |
aOdds for the outcome of groups 2 and 3 compared with group 1
CPR clinical pregnancy rate, EMR early miscarriage rate, LBR-c live birth rate per cycle, P of F chromosomal polymorphisms in only female partners, P of M chromosomal polymorphisms in only male partners, BMI female body mass index, AFC antral follicle count, ET fresh embryo transfer, CET cryopreserved embryo transfer
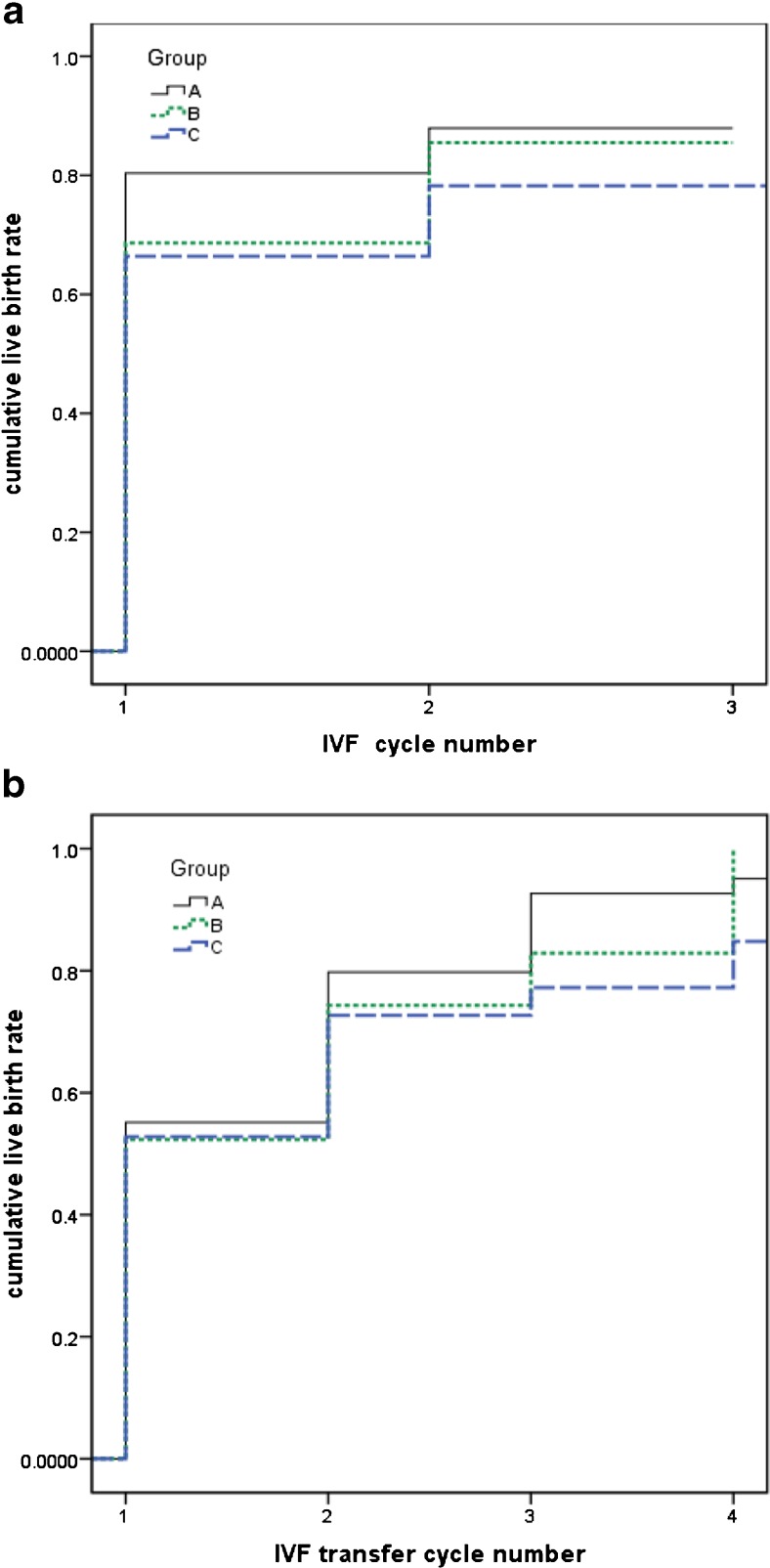
The cumulative effect of chromosomal polymorphisms was illustrated as Fig. 1a, b, which was displayed by transfer cycles and complete cycles separately.
Fig. 1.
a Cumulative live birth rate per woman over multiple complete cycles of IVF. b Cumulative live birth rate per woman over all transfer cycles in a complete IVF cycle
Prevalence of different kinds of chromosomal polymorphisms
As shown in Supplemental Table 2, 1qh+ is the most prevalent in group B with chromosomal polymorphisms in only females (32.6%), followed by 9qh+ (11.6%), and the most common type in D/G group is 21pstk+ and 21ps+ (14.0%). In group C with chromosomal polymorphisms in only males, chromosome Y variations have a comparably high incidence with that of chromosome 1, 9, and 16 variations (36 vs 36.8%). Yqh+ is the most common type (28.8%), followed by 1qh+ (24.8%). Other types with a high incidence are D/G group (25.6%) and Yqh- (7.2%).
Discussion
There is no doubt that chromosomal polymorphisms are correlated with infertility, poor obstetric histories, and spontaneous miscarriage as reported [2–9]. But their impact on outcomes of IVF and ICSI is uncertain, which is possibly ascribed to study populations, different assisted reproductive technology (ART) methods, or analytical procedures in different studies. All subjects in our study are exclusively infertile couples requiring IVF treatment, rather than ICSI, which has never been investigated alone in historical studies regarding the relationship between chromosomal polymorphisms and assisted reproductive outcomes. IVF method excludes couples with severe oligoasthenoteratozoospermia (OAT) and azoospermia, which diminishes the effect of sperm quality on ART outcomes.
In our study, sperm concentration in group C was significantly lower than the control group (group A) in a whole (P = 0.047). This is consistent with the conclusion that chromosomal polymorphisms indeed impact on spermatogenesis, not only in the severe OAT and azoospermia groups [12, 15] but also in the normozoospermia group.
LBR as well as other outcome measures after the first transfer cycle showed no significant difference among these three groups, which is in accordance with historical conclusions within the normozoospermia or oligozoospermia group [11, 12, 16]. Considering that many factors have potential impact on pregnancy outcomes, including sperm concentration mentioned above, then a binary logistic regression model was adopted and yielded the same conclusion. However, the analysis of cumulative pregnancy outcomes demonstrated a significantly higher cumulative BPAR, EMR, and a lower LBR per cycle in couples with chromosomal polymorphisms. And another binary logistic regression model testified the effect of male polymorphisms on cumulative EMR and LBR per cycle. The comparison of Fig. 1a, b resulted from the Kaplan-Meier method vividly explained the cumulative effect of chromosomal polymorphisms.
LBR per woman or per cycle following a single oocyte retrieval is of great clinical significance. This study indicated that couples, with chromosomal polymorphisms in only males, would have a significantly lower LBR after a complete IVF cycle (80.4 vs 68.00%), for a rate ratio of 1.182 (95% CI 1.030 to 1.356, P = 0.01).
Hong Y et al. declared that EMR in the group with male chromosomal polymorphisms, especially in normozoospermia group, was nonsignificantly higher than controls with normal chromosomes (10.31 vs 6.84%, P > 0.05) [11]. Similar with this, but our results further proved that male carriers with normozoospermia would have a significantly higher risk of early miscarriage after cumulative embryo transfer cycles (14.7 vs 7.2%, P = 0.035).
Low LBR and high EMR is probably associated with the various roles of different kinds of polymorphisms. It has been reported that some heterochromatic regions can cause susceptibility to unequal recombination of homologues during cell division and induce chromosomal aberrations such as inversions, deletions, or extensions [17]. And large heterochromatic blocks of different polymorphic types can interfere with the process of chromosomal synapsis and spindle fiber attachment, leading to meiotic errors or arrest [18] and causing gamete and embryo aneuploidy [19]. Yakin K et al. analyzed sperm aneuploidy using fluorescence in situ hybridization (FISH) in 54 infertile men with normal peripheral karyotypes and 8 men with chromosomal polymorphisms and found that there is a prominently high incidence of aneuploidy in sperm of polymorphism carriers, and in this study, the dominant polymorphic types were 9qh+ and 16h+ [15]. In fact, many studies have declared that chromosomal polymorphisms have a higher prevalence with 6–15% in RSA couples [7, 8, 20].
In the present study, Yqh+ is the most common type in male carriers, consistent with previous studies [11, 12]. But the exclusion of males with OAT and azoospermia during IVF treatment might curtail the proportion of Y chromosomal polymorphisms. The variations of the heterochromatic regions on chromosome Y are believed to associate with spermatogenesis by silencing pivotal gene expression and hamper meiosis [5], and increase of chromosome Y and decrease of DYZ1 copy number may be related with RSA or early embryo growth arrest [21]. Xiao Z et al. compared the reproductive outcomes after the first IVF cycle among 72 Yqh+ carriers and 986 Yqh+ non-carriers and found that Yqh+ carriers had a significantly lower fertilization rate (50.05 vs 66.01%, P < 0.05, OR 0.61, CI 0.49–0.57), implantation rate (8.33 vs 20.87%, P < 0.05, OR 0.35, CI 0.14–0.87), and clinical pregnancy rate (17.39 vs 39.59%, P < 0.05, OR 0.32, CI 0.11–0.96) [22]. Nevertheless, some other researchers held the opposite opinion that long Y chromosome or Y chromosome microdeletions display no significant impact on pregnancy outcomes and miscarriage [12, 23–26].
1qh+ and 9qh+ both are dominant chromosomal types in both female carriers and male carriers in this study. Chromosome 9 is highly structurally polymorphic and rich in heterochromatin with DNA satellite repeats (6–8% in human) [27], which is susceptible to hamper the chromosomal pairing. Besides, significant meiotic abnormalities, anomalous aneuploidy rates, and high sperm DNA fragmentation have been observed in an infertile person with a 9qh+++ chromosome by Garcia et al. [28]. And inv(1) was also proved to be interfered with interchromosomal recombination, synapsis, and impair meiosis, inducing sperm aneuploidy [29]. For 1qh+, the most common type found by Nakamura et al. in 1790 infertile men [4] may be associated with miscarriage, but it is uncertain.
Although the current study reports the adverse effect of chromosomal polymorphisms from the angle of LBR and cumulative LBR, the limitations are as follows: first, fertilization rate is not included in this study, considering that this study focuses on pregnancy outcomes and the number of high-quality embryos are proposed to be more relevant; second, this study did not clarify the underlying mechanisms for the cumulative effect of chromosomal polymorphisms; third, large-sample clinical trials in a prospective setting will be needed to further testify our findings.
In conclusion, our results suggested that couples with chromosomal polymorphisms in only male partners have poor pregnancy outcomes after IVF treatment manifesting as high cumulative EMR and low LBR after a complete cycle. Moreover, chromosomal polymorphisms impact spermatogenesis, as evidenced in the normozoospermia group in this study.
Electronic supplementary material
(DOCX 18 kb)
(DOCX 16 kb)
Acknowledgements
The authors would like to thank Keliang Wu and other personnel in the IVF laboratory for their help in precious participation in laboratory procedures.
Compliance with ethical standards
All procedures performed in our study involving human participants were in accordance with the ethical standards of the institutional review board of reproductive medicine and with the 1964 Helsinki Declaration and its later amendments. For this type of study, formal consent is not required. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the National Basic Research Program of China (973 Program) (2012CB944700) and Science Research Foundation Item of No-earnings Health Vocation (201402004).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0951-1) contains supplementary material, which is available to authorized users.
References
- 1.Wyandt HE, Tonk VS. Human chromosome variation: heteromorphism and polymorphism. Berlin: Springer; 2011. [Google Scholar]
- 2.Madon PF, Athalye AS, Parikh FR. Polymorphic variants on chromosomes probably play a significant role in infertility. Reprod BioMed Online. 2005;11(6):726–732. doi: 10.1016/S1472-6483(10)61691-4. [DOI] [PubMed] [Google Scholar]
- 3.Vulkova G. Chromosomal aberrations and chromosomal polymorphism in families with reproductive failure. Folia Med (Plovdiv) 1983;25(3):11–18. [PubMed] [Google Scholar]
- 4.Nakamura Y, Kitamura M, Nishimura K, Koga M, Kondoh N, Takeyama M, et al. Chromosomal variants among 1790 infertile men. Int J Urol. 2001;8(2):49–52. doi: 10.1046/j.1442-2042.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 5.Minocherhomji S, Athalye AS, Madon PF, Kulkarni D, Uttamchandani SA, Parikh FR. A case-control study identifying chromosomal polymorphic variations as forms of epigenetic alterations associated with the infertility phenotype. Fertil Steril. 2009;92(1):88–95. doi: 10.1016/j.fertnstert.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 6.Mierla D, Stoian V. Chromosomal polymorphisms involved in reproductive failure in the Romanian population. Balkan J Med Genet. 2012;15(2):23–28. doi: 10.2478/bjmg-2013-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De la Fuente-Cortes BE, Cerda-Flores RM, Davila-Rodriguez MI, Garcia-Vielma C, De la Rosa Alvarado RM, Cortes-Gutierrez EI. Chromosomal abnormalities and polymorphic variants in couples with repeated miscarriage in Mexico. Reprod BioMed Online. 2009;18(4):543–548. doi: 10.1016/S1472-6483(10)60132-0. [DOI] [PubMed] [Google Scholar]
- 8.Akbas H, Isi H, Oral D, Turkyilmaz A, Kalkanli-Tas S, Simsek S, et al. Chromosome heteromorphisms are more frequent in couples with recurrent abortions. Genet Mol Res. 2012;11(4):3847–3851. doi: 10.4238/2012.November.12.1. [DOI] [PubMed] [Google Scholar]
- 9.Dong Y, Li LL, Wang RX, Yu XW, Yun X, Liu RZ. Reproductive outcomes in recurrent pregnancy loss associated with a parental carrier of chromosome abnormalities or polymorphisms. Genet Mol Res. 2014;13(2):2849–2856. doi: 10.4238/2014.January.17.4. [DOI] [PubMed] [Google Scholar]
- 10.Suganya J, Kujur SB, Selvaraj K, Suruli MS, Haripriya G, Samuel CR. Chromosomal abnormalities in infertile men from southern India. J Clin Diagn Res. 2015;9(7):GC05–GC10. doi: 10.7860/JCDR/2015/14429.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong Y, Zhou YW, Tao J, Wang SX, Zhao XM. Do polymorphic variants of chromosomes affect the outcome of in vitro fertilization and embryo transfer treatment? Hum Reprod. 2011;26(4):933–940. doi: 10.1093/humrep/deq333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T, Qin Y, Gao X, Chen H, Li G, Ma J, et al. The role of male chromosomal polymorphism played in spermatogenesis and the outcome of IVF/ICSI-ET treatment. Int J Androl. 2012;35(6):802–809. doi: 10.1111/j.1365-2605.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Zhang R, Wang W, Liu H, Liu L, Mao B, et al. The effect of chromosomal polymorphisms on the outcomes of fresh IVF/ICSI-ET cycles in a Chinese population. J Assist Reprod Genet. 2016;33(11):1481–1486. doi: 10.1007/s10815-016-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin FI, Yilmaz Z, Yuregir OO, Bulakbasi T, Ozer O, Zeyneloglu HB. Chromosome heteromorphisms: an impact on infertility. J Assist Reprod Genet. 2008;25(5):191–195. doi: 10.1007/s10815-008-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakin K, Balaban B, Urman B. Is there a possible correlation between chromosomal variants and spermatogenesis? Int J Urol. 2005;12(11):984–989. doi: 10.1111/j.1442-2042.2005.01185.x. [DOI] [PubMed] [Google Scholar]
- 16.Liang J, Zhang Y, Yu Y, Sun W, Jing J, Liu R. Effect of chromosomal polymorphisms of different genders on fertilization rate of fresh IVF-ICSI embryo transfer cycles. Reprod BioMed Online. 2014;29(4):436–444. doi: 10.1016/j.rbmo.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Giglio S, Broman KW, Matsumoto N, Calvari V, Gimelli G, Neumann T, et al. Olfactory receptor-gene clusters, genomic-inversion polymorphisms, and common chromosome rearrangements. Am J Hum Genet. 2001;68(4):874–883. doi: 10.1086/319506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lissitsina J, Mikelsaar R, Varb K, Punab M. Cytogenetic study in infertile men. Ann Genet. 2003;46:185. [Google Scholar]
- 19.Morales R, Lledo B, Ortiz JA, Ten J, Llacer J, Bernabeu R. Chromosomal polymorphic variants increase aneuploidies in male gametes and embryos. Syst Biol Reprod Med. 2016;62(5):317–324. doi: 10.1080/19396368.2016.1212949. [DOI] [PubMed] [Google Scholar]
- 20.Caglayan AO, Ozyazgan I, Demiryilmaz F, Ozgun MT. Are heterochromatin polymorphisms associated with recurrent miscarriage? J Obstet Gynaecol Res. 2010;36(4):774–776. doi: 10.1111/j.1447-0756.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 21.Yan J, Fan L, Zhao Y, You L, Wang L, Zhao H, et al. DYZ1 copy number variation, Y chromosome polymorphism and early recurrent spontaneous abortion/early embryo growth arrest. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):371–374. doi: 10.1016/j.ejogrb.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Z, Zhou X, Xu W, Yang J. A preliminary study of the relationship between the long arm of the Y chromosome (Yqh+) and reproductive outcomes in IVF/ICSI-ET. Eur J Obstet Gynecol Reprod Biol. 2012;165(1):57–60. doi: 10.1016/j.ejogrb.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Pereza N, Crnjar K, Buretic-Tomljanovic A, Volk M, Kapovic M, Peterlin B, et al. Y chromosome azoospermia factor region microdeletions are not associated with idiopathic recurrent spontaneous abortion in a Slovenian population: association study and literature review. Fertil Steril. 2013;99(6):1663–1667. doi: 10.1016/j.fertnstert.2013.01.101. [DOI] [PubMed] [Google Scholar]
- 24.Liu XH, Qiao J, Li R, Yan LY, Chen LX. Y chromosome AZFc microdeletion may not affect the outcomes of ICSI for infertile males with fresh ejaculated sperm. J Assist Reprod Genet. 2013;30(6):813–819. doi: 10.1007/s10815-013-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghorbian S, Saliminejad K, Sadeghi MR, Javadi GR, Kamali K, Amirjannati N, et al. The association between Y chromosome microdeletion and recurrent pregnancy loss. Iran Red Crescent Med J. 2012;14(6):358–362. [PMC free article] [PubMed] [Google Scholar]
- 26.Nie H, Lu G. Long Y chromosome is not a fetal loss risk. J Assist Reprod Genet. 2011;28(2):151–156. doi: 10.1007/s10815-010-9497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphray SJ, Oliver K, Hunt AR, Plumb RW, Loveland JE, Howe KL, et al. DNA sequence and analysis of human chromosome 9. Nature. 2004;429(6990):369–374. doi: 10.1038/nature02465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Peiro A, Oliver-Bonet M, Navarro J, Abad C, Guitart M, Amengual MJ, et al. Sperm DNA integrity and meiotic behavior assessment in an infertile male carrier of a 9qh+++ polymorphism. J Biomed Biotechnol. 2011;2011:730847. doi: 10.1155/2011/730847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkpatrick G, Chow V, Ma S. Meiotic recombination, synapsis, meiotic inactivation and sperm aneuploidy in a chromosome 1 inversion carrier. Reprod BioMed Online. 2012;24(1):91–100. doi: 10.1016/j.rbmo.2011.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)
(DOCX 16 kb)