Figure 1.
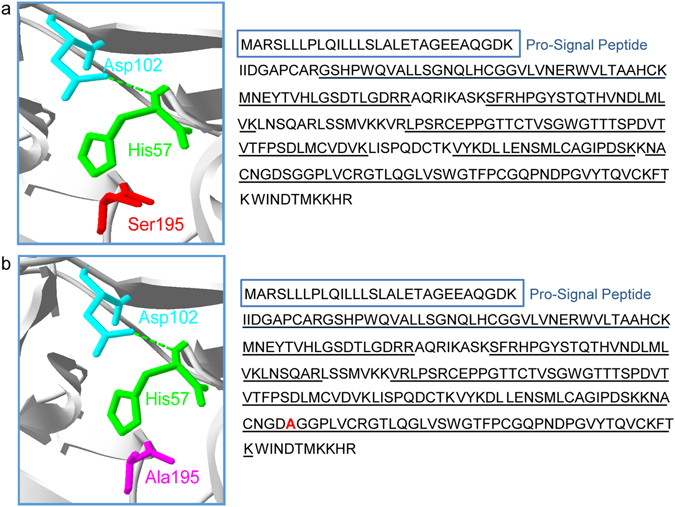
KLK7 and mKLK7 protein identity. (a) Surface exposed residues corresponding to the catalytic triad (His57, Asp102 and Ser195; Schechter and Berger notation12) of KLK7 were identified on the three dimensional (3D) structure available in Protein Data Bank (PDB, accession: 2QXG.pdb in standard serine protease orientation) using SPDBV v4.10. (b) In addition, S195A mutation was made using the mutation tool on SPDBV v4.10. Alongside, are the peptides identified in the MS analysis (Underlined; identified with a 99% protein probability and 95% peptide probability cut off) of trypsin-digested (a) active KLK7 and (b) mKLK7 aligned with the KLK7 full length protein (UniProtKB; P49862-1). The pro-signal peptide (in blue box) was not included in the expression construct, thus obtaining mature amino acid sequences for these peptidases. Expected mutation, S195A was identified in the MS-identified mKLK7 peptide sequence as highlighted in red bold letter.
