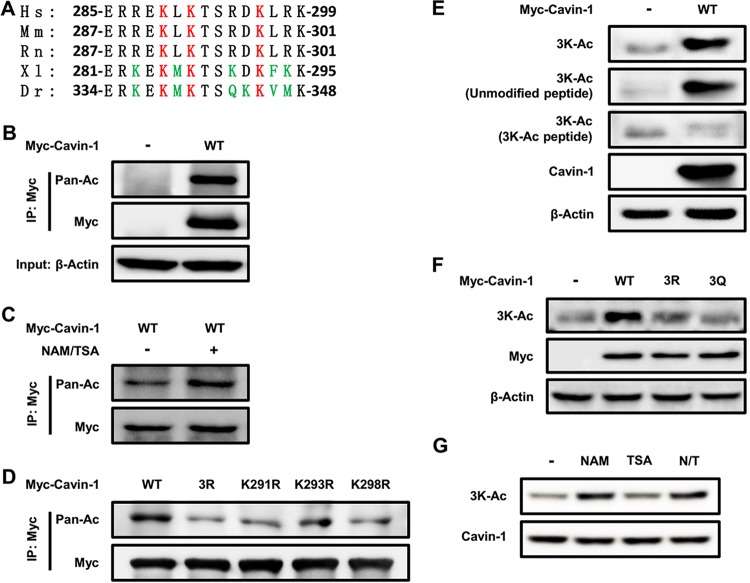
FIG 1.
Acetylation of cavin-1 at lysines 291, 293, and 298. (A) The identified acetylated K291, K293, and K298 (Ac-3K) in cavin-1 are conserved. Multiple alignments of the protein sequences around 3K (lysines 291, 293, and 298)-acetylated sites of cavin-1 identified by mass spectrometry from different organisms. Hs, Homo sapiens (human); Mm, Mus musculus (mouse); Rn, Rattus norvegicus (Norway rat); Xl, Xenopus laevis (African clawed frog); Dr, Danio rerio (zebrafish). (B and C) Exogenous cavin-1 is acetylated. Myc-tagged cavin-1 was transfected into HEK293T cells with or without nicotinamide (NAM) and trichostatin A (TSA) treatment and immunoprecipitated for acetylation analysis by Western blotting with either an anti-pan-acetyl lysine or an anti-Myc antibody. (D) Mutation of K291, K293, K298, or 3K decreases cavin-1 acetylation. HEK293T cells were transfected with the indicated plasmids, and cavin-1 acetylation and protein levels were analyzed by Western blotting with the indicated antibodies. (E and F) The anti-3K-Ac antibody is specific for K291, K293, and K298 of cavin-1. (E) The indicated plasmids were transfected into HEK293T cells. The specificity of the anti-3K-Ac antibody was determined by Western blotting; the antibody was preincubated with or without antigen peptides (acetyl-3K peptide or unmodified peptide). (F) Acetylation of Myc-tagged cavin-1WT, cavin-13R, and cavin-13Q was measured by Western blotting with an anti-3K-Ac antibody. (G) NAM, but not TSA, increases 3K acetylation of cavin-1. 3T3-L1 adipocytes were left untreated or were treated with sirtuin deacetylase inhibitor NAM and histone deacetylase inhibitor TSA. Acetylation of endogenous cavin-1 was determined by Western blotting with an anti-3K-Ac antibody. N/T, treated with NAM and TSA.