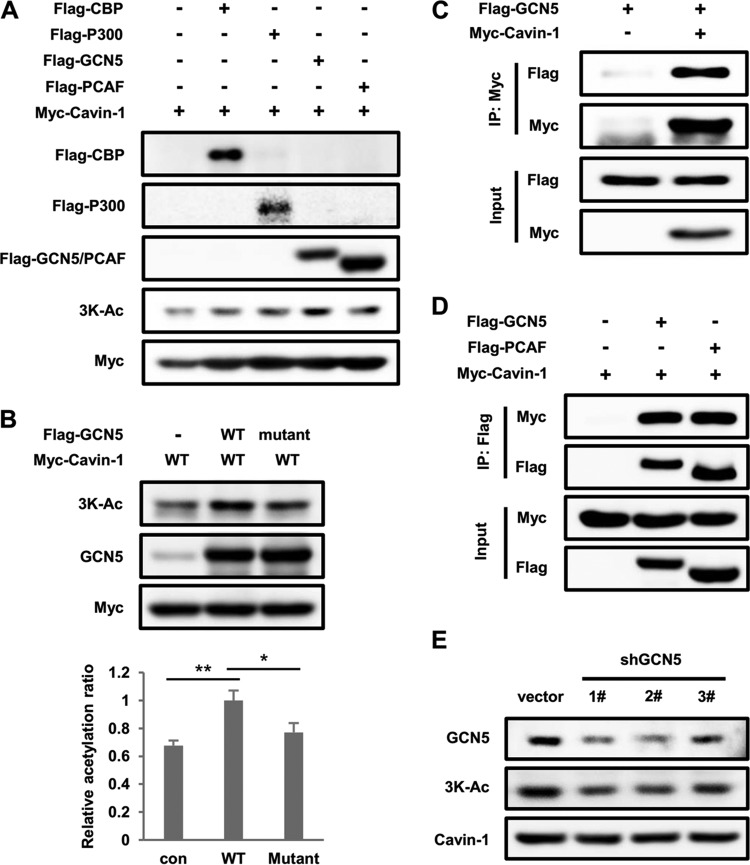
FIG 3.
GCN5 enhances cavin-1 acetylation. (A) Overexpression of GCN5 but not other acetyltransferases increases cavin-1 acetylation at 3K. Myc-tagged cavin-1 was transfected into HEK293T cells with Flag-tagged CBP, P300, GCN5, or PCAF. Cavin-1 acetylation was measured by Western blotting with an anti-3K-Ac antibody. (B) A catalytically inactive GCN5 mutant does not acetylate cavin-1. HEK293T cells were transfected with the indicated plasmids, and acetylation levels of cavin-1 were analyzed by Western blotting. Relative acetylation/protein ratios were quantified. con, cotransfected with vector control; WT, cotransfected with wild-type GCN5; Mutant, cotransfected with the catalytically inactive GCN5 mutant. Error bars represent ±SD for experiments performed in triplicate. (C) GCN5 binds with cavin-1. Myc-tagged cavin-1 was cotransfected with Flag-tagged GCN5 into HEK293T cells and immunoprecipitated with an anti-Myc antibody. The presence of GCN5 in immunoprecipitates was determined by Western blotting with an antibody against Flag. (D) Cavin-1 coimmunoprecipitates with GCN5 and PCAF. The indicated plasmids were coexpressed into HEK293T cells, and the interactions of cavin-1 with GCN5 and PCAF were analyzed by immunoprecipitation and Western blotting. (E) GCN5 knockdown decreases endogenous cavin-1 acetylation at 3K. 3T3-L1 adipocytes were transduced with lentivirus-driven shGCN5 or control shRNA, and acetylation of endogenous cavin-1 was measured by Western blotting using an anti-3K-Ac antibody.