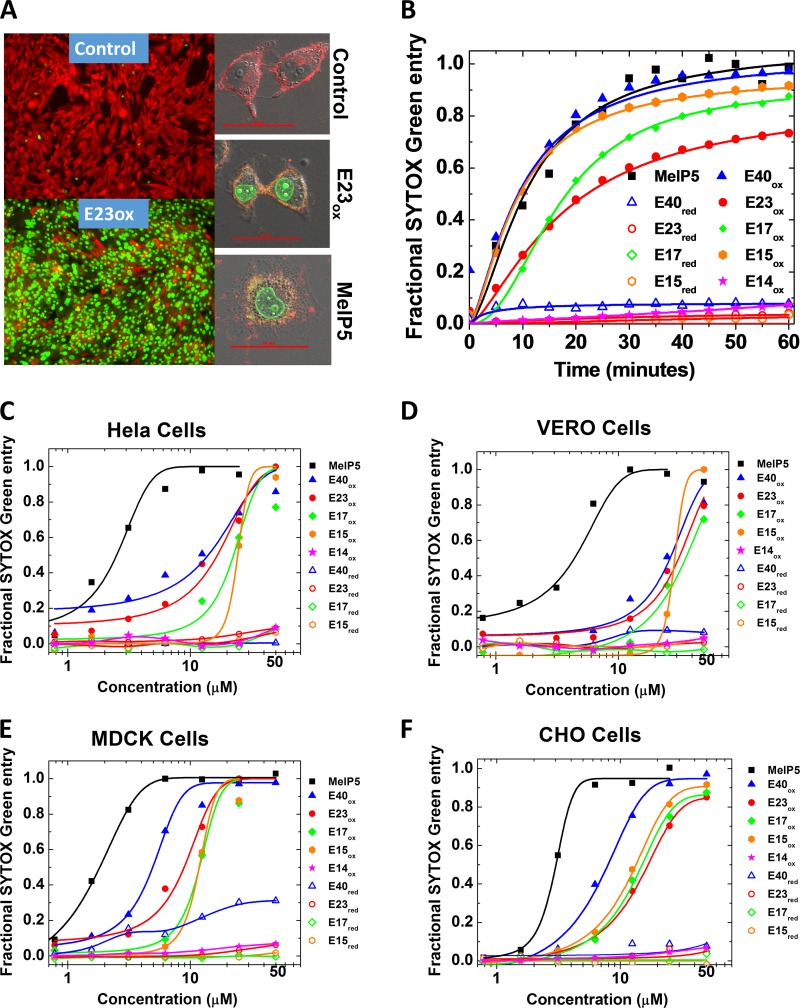
FIG 3.
Permeabilization of cell membranes by delta peptides. (A) (Left) Example epifluorescence images of cells incubated with CAMRO and Sytox green. CAMRO enters the cytosol, where it is converted to the fluorescent and membrane-impermeant dye (red). Sytox green is a membrane-impermeant DNA-binding dye that becomes fluorescent only if it can access the nucleus due to plasma membrane disruption. Intact cells are red, and cells permeable to both dyes are green. The lower image shows cells treated with 50 μM E23ox for 1 h. (Right) Example confocal microscopy images of cells labeled with rhodamine-18, which labels membranes, and Sytox green, which fluoresces in the nucleus if cells are permeabilized. The cells were treated with buffer only, 40 μM E23ox, or 5 μM MelP5. Scale bars, 50 μm. (B) Time dependence of peptide-induced plasma membrane permeabilization of CHO cells as measured by Sytox green fluorescence. Delta peptides are all present at 25 μM and MelP5 is present at 5 μM. (C to F) Sytox green experiments for all cell types studied. Cells were seeded into 96-well tissue culture plates and grown to 80 to 90% confluence for 24 to 48 h in full medium. Five minutes before the addition of peptide, 100 nM Sytox green was added. The peptides were first serially diluted and then added to the cells. Sytox green fluorescence at 60 min was used to calculate fractional permeabilization of the plasma membrane. The curves are fitted with a sigmoidal function to determine the EC50 from the curve midpoint.