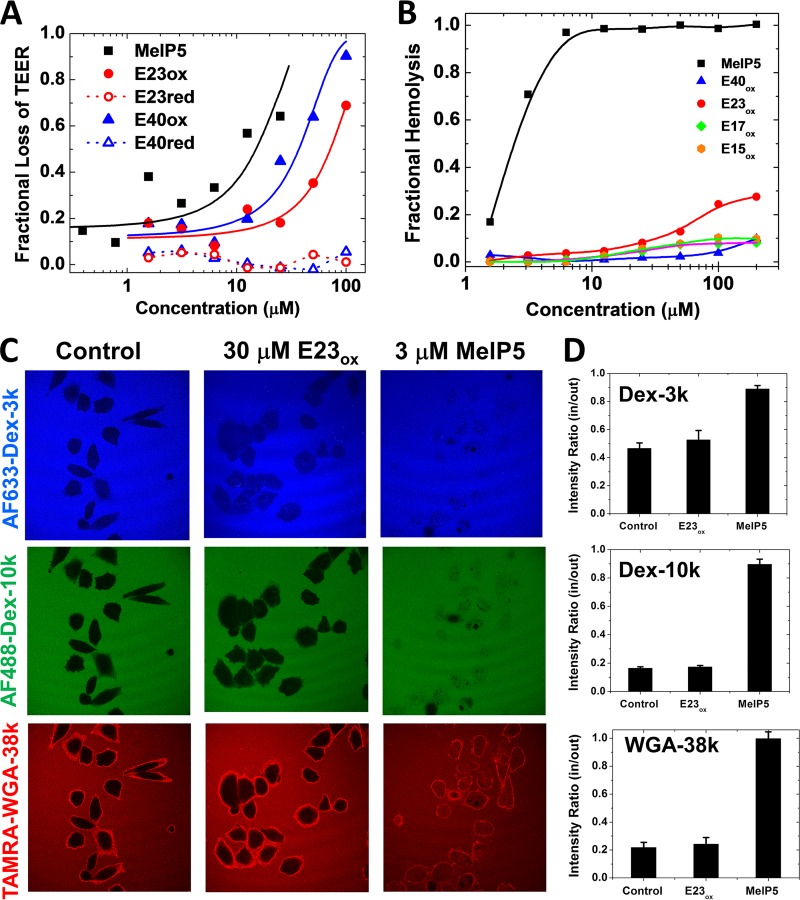
FIG 4.
Mechanism of cell permeabilization. (A) Peptide-induced loss of transepithelial electrical resistance in a high-resistance, confluent monolayer of MDCK cells. The cells were treated with peptides, and TEER was measured for 1 h. Resistance at 60 min minus background resistance without cells is plotted as a fraction of the initial resistance minus background. In all experiments, the control for 100% cell permeabilization was MelP5. (B) Lysis of human erythrocytes. Fresh human red blood cells at 2 × 108 RBC/ml were incubated with serially diluted peptides. After incubation for 1 h at 37°C, the cells were centrifuged and the released hemoglobin in the supernatant was measured by optical absorbance of the heme group (410 nm). The negative control was buffer only (0% lysis), and the positive control was distilled water (100% lysis). While MelP5 is lytic at 1 to 3 μM, even 200 μM delta peptides cause only slight hemolysis. (C) Peptide-induced entry of macromolecules. CHO cells were simultaneously incubated with Alexa Fluor 633-dextran-3k (blue), Alexa Fluor 488-dextran-10k (green), and 6-carboxytetramethylrhodamine (TAMRA)-wheat germ agglutinin (WGA) (39 kDa; red). Buffer or peptides at 2 times the EC50 were added, and the cells were incubated for 1 h, followed by imaging by fluorescence scanning confocal microscopy. (D) Intensities inside and outside cells were measured with ImageJ for 20 to 25 cells in at least two independent fields. The error bars indicate standard deviations (SD).