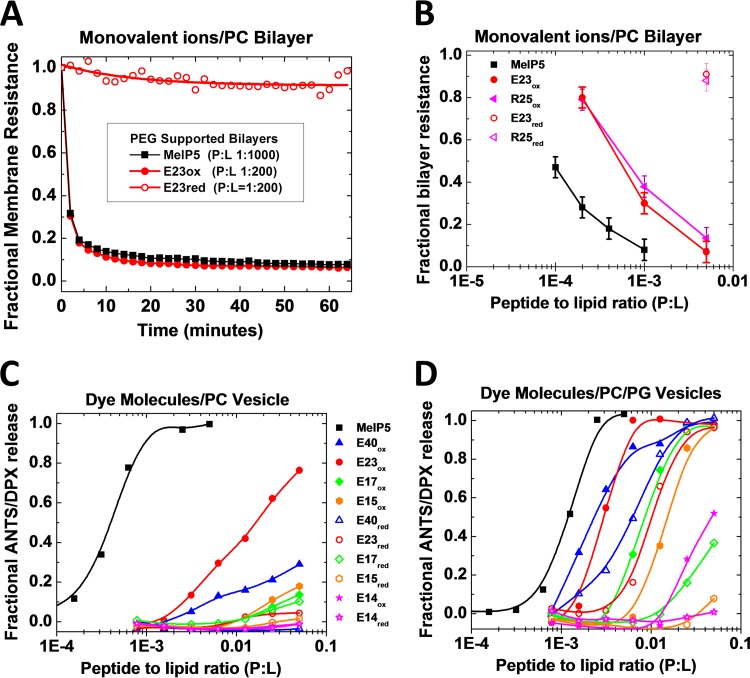
FIG 5.
Permeabilization of synthetic bilayers. (A) Electrochemical impedance spectroscopy using polymer-cushioned, surface-supported PC bilayers made by Langmuir-Blodgett deposition on single-crystal silicon. Bilayer resistance is determined by fitting an equivalent circuit to spectra collected at 1-min intervals after addition of peptide at time zero. Peptide-to-lipid ratios are for the whole system, including vesicles in solution that are in equilibrium with the surface-supported bilayer. Resistance, in this experiment, is based on the movement of monovalent ions, such as Na+, Cl−, H+, and OH–, or Ca2+ and Mg2+, across the bilayer. (B) Fractional loss of supported bilayer resistance versus peptide concentration. The values are for measurements made 60 min after addition of peptide. (C and D) Lipid vesicle permeabilization. Large unilamellar vesicles were made by extrusion from POPC (C) and POPC plus 50% POPG (D). Vesicles contained the dye ANTS (6 mM) and its quencher, DPX (12 mM). Peptide serial dilutions were added to 0.5 mM lipid vesicles in the wells of a 96-well plate. After 1 h, ANTS fluorescence was measured, and fractional leakage was calculated relative to buffer only (0% leakage) and Triton X-100 or MelP5 at a P/L ratio of 1:50 (100% leakage). The curves are fitted with a sigmoidal function to determine EC50 from the curve midpoint.