Abstract
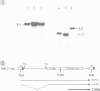
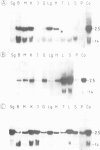
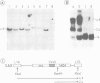
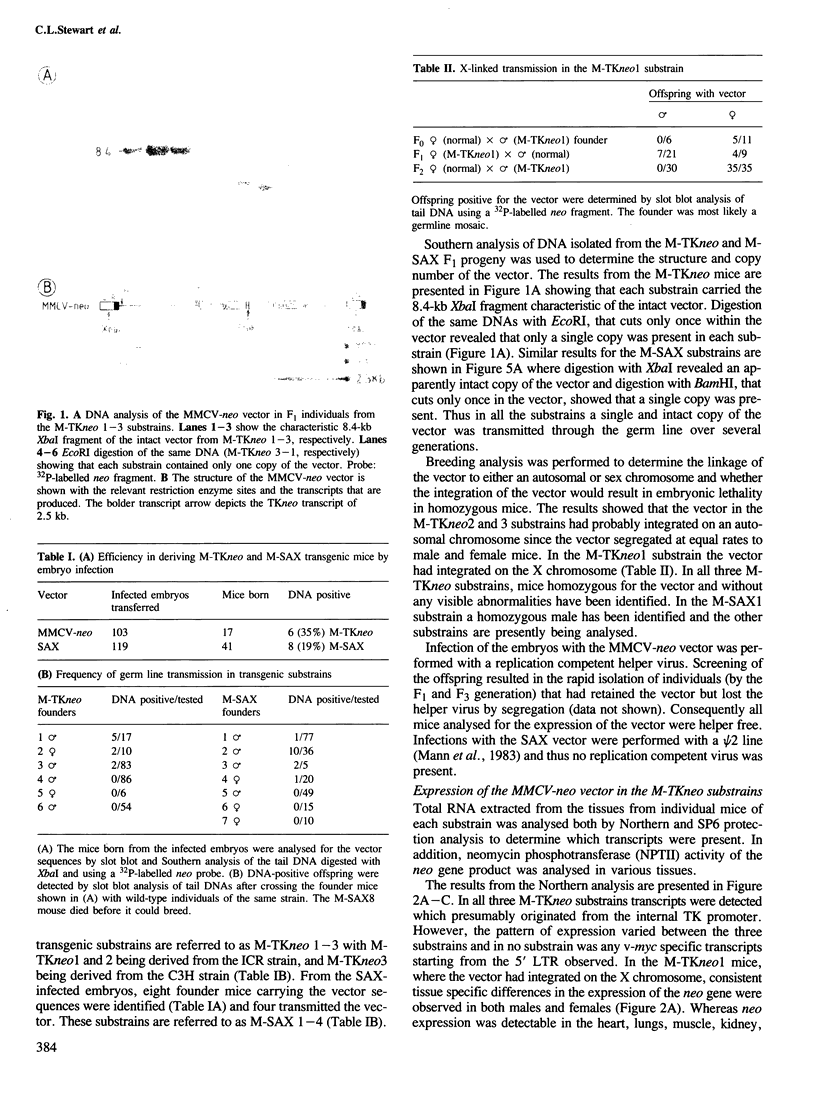
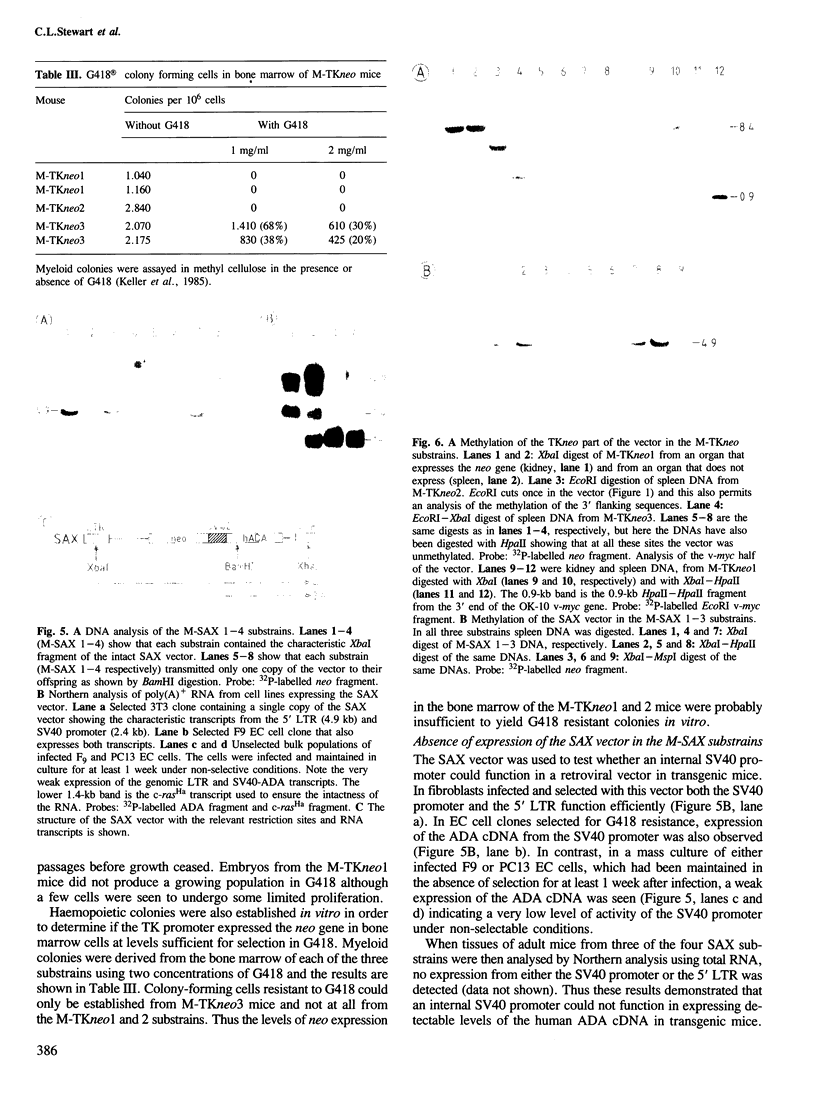
Pre-implantation embryos were infected with the retroviral vector MMCV-neo, which carries the neomycin resistance (neo) gene and the v-myc gene. Three transgenic substrains (M-TKneo 1-3) were derived which stably transmit a single intact copy of the vector. In all of the substrains, expression of the neo gene from the internal thymidine kinase (TK) promoter was detected, with two of the substrains expressing the gene in all tissues analysed. In the third substrain, the vector had integrated on the X chromosome and neo expression varied between different tissues. A second series of transgenic mice were obtained with the retroviral vector SAX, in which the human adenosine deaminase cDNA (ADA) is under the control of an internal SV40 promoter. Four substrains (M-SAX 1-4) were analysed; however, no expression of the ADA cDNA was detected. In all mice, no expression was found of the genes under the control of the viral 5' long terminal repeats (LTRs). In the M-TKneo substrains the vector was hypomethylated irrespective of its expression whereas in the M-SAX mice the vector was hypermethylated. These results demonstrate for the first time that the TK promoter can apparently express a gene in all tissues of adult mice and that retroviral vectors with internal promoters may provide an alternative to DNA injection for the efficient expression of genes in transgenic mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinster R. L., Chen H. Y., Messing A., van Dyke T., Levine A. J., Palmiter R. D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984 Jun;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T. D., Lehman J. M. The state of simian virus 40 DNA in the embryonal carcinoma cells of the murine teratocarcinoma. Virology. 1981 Apr 15;110(1):159–166. doi: 10.1016/0042-6822(81)90017-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A., Wagner H., Werner E., Simon D. Complete DNA methylation does not prevent polyoma and simian virus 40 virus early gene expression. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6470–6474. doi: 10.1073/pnas.80.21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D., Balling R., Kothary R., Magli M. C., Hozumi N., Rossant J., Bernstein A. Insertion of a bacterial gene into the mouse germ line using an infectious retrovirus vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8587–8591. doi: 10.1073/pnas.82.24.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Breindl M., Harbers K., Jähner D., Löhler J. Retroviruses and insertional mutagenesis. Cold Spring Harb Symp Quant Biol. 1985;50:439–445. doi: 10.1101/sqb.1985.050.01.055. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D. Methylation, expression and chromosomal position of genes in mammals. Biochim Biophys Acta. 1984 May 15;782(1):1–9. doi: 10.1016/0167-4781(84)90099-x. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jähner D., Haase K., Mulligan R., Jaenisch R. Insertion of the bacterial gpt gene into the germ line of mice by retroviral infection. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6927–6931. doi: 10.1073/pnas.82.20.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Stewart C. L., Harbers K., Löhler J., Simon I., Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- Kantoff P. W., Kohn D. B., Mitsuya H., Armentano D., Sieberg M., Zwiebel J. A., Eglitis M. A., McLachlin J. R., Wiginton D. A., Hutton J. J. Correction of adenosine deaminase deficiency in cultured human T and B cells by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6563–6567. doi: 10.1073/pnas.83.17.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Chapman V. M., Hammer R. E., Brinster R., Tilghman S. M. Differential expression of alpha-fetoprotein genes on the inactive X chromosome in extraembryonic and somatic tissues of a transgenic mouse line. Nature. 1986 Jan 16;319(6050):224–226. doi: 10.1038/319224a0. [DOI] [PubMed] [Google Scholar]
- Levis R., Hazelrigg T., Rubin G. M. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science. 1985 Aug 9;229(4713):558–561. doi: 10.1126/science.2992080. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O. Suppression of the hypomethylated Moloney leukemia virus genome in undifferentiated teratocarcinoma cells and inefficiency of transformation by a bacterial gene under control of the long terminal repeat. Mol Cell Biol. 1985 Sep;5(9):2325–2331. doi: 10.1128/mcb.5.9.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Messing A., Brinster R. L. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumours in transgenic mice. Nature. 1985 Aug 1;316(6027):457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Construction of a retrovirus capable of transducing and expressing genes in multipotential embryonic cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7137–7140. doi: 10.1073/pnas.81.22.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Introduction of genes into preimplantation mouse embryos by use of a defective recombinant retrovirus. Proc Natl Acad Sci U S A. 1986 Jan;83(2):366–368. doi: 10.1073/pnas.83.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholnick S. B., Morgan B. A., Hirsh J. The cloned dopa decarboxylase gene is developmentally regulated when reintegrated into the Drosophila genome. Cell. 1983 Aug;34(1):37–45. doi: 10.1016/0092-8674(83)90134-4. [DOI] [PubMed] [Google Scholar]
- Soriano P., Jaenisch R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell. 1986 Jul 4;46(1):19–29. doi: 10.1016/0092-8674(86)90856-1. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Rüther U., Garber C., Vanek M., Wagner E. F. The expression of retroviral vectors in murine stem cells and transgenic mice. J Embryol Exp Morphol. 1986 Oct;97 (Suppl):263–275. [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L., Vanek M., Wagner E. F. Expression of foreign genes from retroviral vectors in mouse teratocarcinoma chimaeras. EMBO J. 1985 Dec 30;4(13B):3701–3709. doi: 10.1002/j.1460-2075.1985.tb04138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Cone R., Mulligan R. C., Jaenisch R. Introduction of a selectable gene into different animal tissue by a retrovirus recombinant vector. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7151–7155. doi: 10.1073/pnas.81.22.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Keller G., Gilboa E., Rüther U., Stewart C. Gene transfer into murine stem cells and mice using retroviral vectors. Cold Spring Harb Symp Quant Biol. 1985;50:691–700. doi: 10.1101/sqb.1985.050.01.085. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H., Botteri F. M., Miller A. D., Rosenfeld M. G., Fan H., Evans R. M., Verma I. M. Efficient insertion of genes into the mouse germ line via retroviral vectors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6148–6152. doi: 10.1073/pnas.82.18.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]