Abstract
BACKGROUND
Several studies demonstrated that intraoperative near-infrared fluorescence (NIRF) imaging using indocyanine green (ICG) identifies (sub)capsular colorectal liver metastases (CRLM) missed by other techniques. It is unclear if this results in any survival benefit. This study evaluates long-term follow-up after NIRF-guided resection of CRLM using ICG.
METHODS
First, patients undergoing resection of CRLM with or without NIRF imaging were analyzed retrospectively. Perioperative details, liver-specific recurrence-free interval and overall survival were compared. Second, the prognosis of patients in whom additional metastases were identified solely by NIRF was studied.
RESULTS
Eighty-six patients underwent resection with NIRF imaging and 87 without. In significantly more patients of the NIRF imaging cohort additional metastases were identified during surgery (25% vs. 13%, p=0.04). Tumors identified solely by NIRF imaging were significantly smaller compared to additional metastases identified also by inspection, palpation or intraoperative ultrasound (3.2 ± 1.8 mm vs. 7.4 ± 2.6 mm, p<0.001). Liver-specific recurrence-free survival at 4 years was 47% with NIRF imaging and 39% without (hazard ratio at multivariate analysis 0.73, 95%CI 0.42–1.28, p=0.28). Overall survival at 4 years was 62% and 59%, respectively (p=0.79). No liver recurrences occurred within 3 years follow-up in 52% of patients in whom additional metastases were resected based on only NIRF imaging.
CONCLUSIONS
This study suggests that NIRF imaging identifies significantly more and smaller tumors during resection of CRLM, preventing recurrences in a subset of patients. Given its safety profile and low expense, routine use can be considered until tumor targeting fluorescent tracers are clinically available. Keywords: .
Keywords: indocyanine green, cancer, liver neoplasms, surgery, prognosis, fluorescence imaging
INTRODUCTION
Intrahepatic recurrence rates after resection of colorectal liver metastases (CRLM) remain high, despite improvements in preoperative imaging modalities and chemotherapy regimens.[1–3] The majority of patients develop recurrence within 12 months, suggesting that tumors were missed previously.[4] Indeed, current pre- and intraoperative imaging techniques have low sensitivity for subcentimeter lesions in the liver.[5–7] Near-infrared fluorescence (NIRF; wavelengths 700–900 nm) imaging with targeted or non-targeted fluorescent tracers empowers surgeons to improve contrast between structures and aids in differentiating malignant from benign tissue.[8] In general, NIRF imaging has the potential to improve clinical outcomes by improving radical resection rates and visualizing more malignant lesions, but most published studies are proof-of-concept designs. To date, randomized controlled trials have been performed only with 5-aminolevulinic acid (5-ALA) to guide brain surgery.[9] 5-ALA leads to intracellular accumulation of fluorescent protoporphyrin IX (emission peak at 635 nm, outside NIR) in malignant gliomas. Actually, the benefits of 5-ALA were evident in observational studies even before the start of a randomized controlled trial. Fluorescence imaging of malignant glioma had 85% sensitivity and 100% specificity, overall survival (OS) strongly correlated with residual intraoperative fluorescence, and no safety issues were recorded.[10, 11] Not unexpectedly, the randomized study had to be terminated prematurely due to 20% higher progression-free survival in the 5-ALA arm at 6 months.
Parallels can be drawn between 5-ALA for glioma surgery and indocyanine green (ICG, emission peak at 820 nm) for resection of CRLM, even though the diseases and consequences are different. NIRF imaging using ICG identifies (sub)capsular micrometastases missed by conventional modalities in up to 17% of CRLM patients.[12–17] In addition, screening of resection margins can detect residual tumor, enabling complete removal of all tumor tissue (R0 resection).[17, 18] Intravenous administration of ICG is safe; adverse reactions are reported in less than 1 in 40,000 patients.[19] ICG is widely used for clinical applications (e.g. to test liver function prior to major liver surgery).
It seems unjustifiable to randomize patients into a control arm without NIRF imaging when previous studies have already shown that NIRF imaging identifies additional tumors in the context of an excellent safety profile. However, before NIRF imaging can be accepted and implemented in routine clinical practice, the optical imaging community must show long-term benefits, while also addressing safety and cost-effectiveness.[20] A key unanswered question is whether the (micro)metastases additionally identified by NIRF are indicative of otherwise undetectable, widespread metastases in the liver or if patients are in fact cured by resecting these lesions. This multicenter study is the first to report long-term follow-up after NIRF-guided resection of CRLM. In addition, perioperative data and post-operative outcomes were compared with a cohort of patients that underwent resection of CRLM without intraoperative NIRF imaging.
PATIENTS AND METHODS
Patients
All patients undergoing resection of CRLM with or without NIRF imaging at Leiden University Medical Center (LUMC, Leiden, the Netherlands) between January 2010 and June 2016 were included and termed ‘analysis 1’. Patients received ICG and underwent NIRF imaging only if a NIRF imaging system and operator were available, if patients were willing to participate and if none of the exclusion criteria was met. Exclusion criteria consisted of contraindications for ICG: eGFR < 55; pregnancy; breastfeeding; hyperthyroidism; or an allergy to iodine, shellfish, or ICG. Patients with an eGFR < 55 or hyperthyroidism that underwent resection of CRLM without NIRF imaging were excluded to prevent selection bias. The local institutional review board approved the studies. All patients receiving ICG provided informed consent. Patients with a prior history of metastatic disease were excluded from analysis. Demographics, patient characteristics, Fong’s clinical risk score for predicting recurrence after hepatic resection of CRLM [21], perioperative and long-term follow-up data were collected. Patients were divided into 2 cohorts: a control cohort that underwent standard resection and an experimental cohorts that underwent NIRF-guided resection.
Intraoperative NIRF imaging (analysis 1)
Patients in the NIRF cohorts received a dose of 10 or 20 mg ICG 1 or 2 days prior to surgery. Four different NIRF imaging systems were used: Mini-FLARE® (Frangioni Laboratory, Harvard Medical School, Boston, MA, U.S., see Figure 1)[22], Kit-FLARE® (FLARE Foundation, defunct, previously Wayland, U.S.), Artemis (Quest Innovations BV, Middenmeer, the Netherlands)[23], and Storz HD laparoscope (KARL STORZ GmbH & Co. KGm, Tuttlingen, Germany).
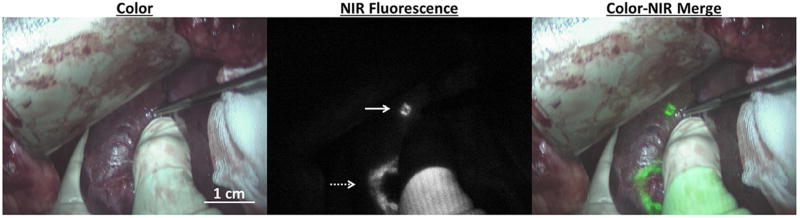
Figure 1. Intraoperative near-infrared fluorescence imaging of liver metastases.
A liver metastasis identified by NIRF imaging only (white arrow) in a patient that received 10 mg indocyanine green one day prior to surgery. Tumors that were already identified by preoperative imaging (dashed arrow) can be demarcated by fluorescence imaging. Imaging was performed using the Mini-FLARE®.
Preoperative workup and surgical procedure (analysis 1)
All patients underwent computed tomography (CT) to detect hepatic and/or extrahepatic metastases. In selected cases, when deemed necessary by the medical team, magnetic resonance imaging (MRI) or positron emission tomography (PET) was performed. The protocol for imaging of CRLM was updated several times and new scanners were acquired by the hospitals during the period 2010–2016. Since 2015 Primovist-enhanced and diffusion weighted MRI was performed. Chemotherapy treatment was divided into 3 categories: (1) no chemotherapy, (2) neoadjuvant chemotherapy was defined as chemotherapy treatment specifically aimed to decrease hepatic tumor load prior to the planned liver surgery and (3) adjuvant chemotherapy as chemotherapy treatment with curative intent aimed at reducing recurrence rate of CRLM following liver resection.
Surgery started with inspection and palpation (the latter during open surgery only), followed by intraoperative ultrasound (IOUS) performed by a dedicated radiologist. Subsequently, the accessible liver surface of patients in the experimental cohorts was screened by NIRF imaging. Note that with the currently available systems, only the accessible surface of the liver was interrogated. Metastases were considered additionally identified if they were not detected by any type of preoperative imaging. They were classified by their method of detection: standard (inspection, palpation and/or IOUS) or NIRF imaging alone. Intraoperative radiofrequency ablation (RFA) was – if deemed necessary – performed under IOUS-guidance by the interventional radiologist. Hemihepatectomy was defined by resection of liver segments 2, 3 and 4 or 5, 6, 7 and 8.
Perioperative details, including length of surgery, resected volume, reoperation rates and complications, were analyzed. The tumor volume was estimated by calculating the spherical volume using the maximum diameter of the resected tumor. Histopathological examination was considered the golden standard. When no tissue was obtained, e.g. if RFA was performed, the diagnosis was based on imaging.
Follow-up (analysis 1)
A CT was performed with 4 months intervals in the first 2 years after surgery. If intraoperative RFA was performed an additional CT was performed directly after surgery. In case patients were disease-free at 2 years follow-up, assessment by CT was done each 6 months for an additional period of 3 years, after which the follow-up ended in case of no recurrence. Additional MRI and PET were used at the discretion of the specialist. The diagnosis of recurrence and/or progression was based on available radiologic imaging. Recurrences were divided into hepatic and extrahepatic recurrence.
Survival after resection of metastases identified solely by NIRF imaging (analysis 2)
An additional cohort, termed ‘analysis 2’, was created to answer the question whether the (micro)metastases additionally identified by NIRF are indicative of otherwise undetectable, widespread metastases in the liver or if patients are in fact cured by resecting these lesions. Only patients in whom additional lesions were identified solely by NIRF imaging were included. To increase statistical power, also patients who underwent NIRF-guided resection of CRLM at the San Matteo General Hospital (SMGH, Pavia, Italy) between August 2011 and June 2012 were included. Due to significant differences in baseline, adjuvant treatment protocols and follow-up, these patients could not be included in analysis 1. At the SMGH patients received a dose of 0.5 mg/kg ICG and the PhotoDynamic Eye (PDE, Hamamatsu Photonics K.K., Hamamatsu-city, Japan) was used. SMGH patients received an ultrasound at 3 months and a CT at 6 months follow-up. Subsequently, CT was performed annually for up to three years.
Statistical analyses
Baseline characteristics between cohorts were compared using an unpaired samples T-test for parametric continuous data, Mann-Whitney U-test for non-parametric continuous data and a Pearson’s Chi-square test for dichotomous or categorical variables. Baseline characteristics of patients treated with fluorescence imaging at the LUMC and SMGH were compared with the cohort of patients treated without fluorescence imaging at the LUMC. Parametric continuous data was reported as mean ± standard deviation (SD), non-parametric continuous data as median with range, and dichotomous or categorical variables as percentage. Liver-specific recurrence-free interval was considered the main outcome of this study as NIRF imaging using ICG can only be used to identify metastases located in the liver. All interval and survival curves were estimated using the Kaplan- Meier method. Long-term results were analyzed until only 25% of all cases were left. Multivariate regression was performed using to the Cox proportional hazards model. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS® version 23; IBM, IL, USA). A pvalue of <0.05 was considered statistically significant.
RESULTS
Patient Demographics (analysis 1 & 2)
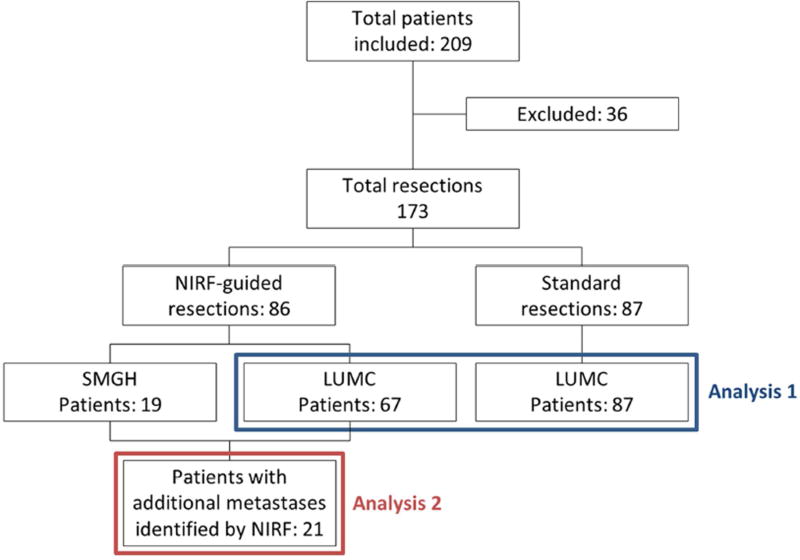
A total of 209 patients was analyzed (Figure 2) of which 36 were excluded either due to a history of metastatic disease (n=7), no resection of metastases (n=15) or eGFR <55 (n=8; i.e. exclusion criterion for ICG administration). For 6 patients at the SMGH no follow-up was available. These patients were consequently also excluded. Baseline characteristics are presented in Table 1. Sixty-seven patients were included in the LUMC experimental cohort and 87 in the LUMC control cohort. The Mini-FLARE® was used in 43 patients, Kit-FLARE® in 1 patient, Artemis in 13 patients, and Storz HD laparoscope in 10 patients. No significant differences in baseline characteristics were observed between the LUMC experimental cohort and LUMC control cohort. Intraoperative results of some of the included patients have been reported previously.[15–17, 24] At the LUMC, adjuvant chemotherapy was no longer standard-of-care since 2013, resulting in a significant difference between the LUMC and SMGH experimental cohorts. Furthermore, patients from SMGH had more comorbidity, including COPD, diabetes and cardiovascular events, resulting in a significantly higher ASA score.
Figure 2. Patient selection.
NIRF: near-infrared fluorescence; LUMC: Leiden University Medical Center; SMGH: San Matteo General Hospital. Analysis 1 assesses differences in perioperative details and survival between patients from LUMC who underwent surgery with or without guidance by near-infrared fluorescence. Analysis 2 includes only patients in whom additional metastases were identified solely by near-infrared fluorescence imaging.
Table 1.
Baseline characteristics
| Cohorts for analysis 1 |
Comparison (p-value) |
Additional patients for analysis 2 |
||
|---|---|---|---|---|
| LUMC Control % (n=87) |
LUMC NIRF % (n=67) |
LUMC Control vs NIRF (n=154) |
SMGH NIRF % (n=19) |
|
| Gender | 0.33 | |||
| Female | 36 (31/87) | 43 (29/67) | 42 (8/19) | |
| Male | 64 (56/87) | 57 (38/67) | 58 (11/19) | |
| Age at surgery, mean ± SD (n) | 63 ± 9.4 (87) | 62 ± 9.2 (67) | 0.55 | 61 ± 9.3 (19) |
| Chemotherapy | 0.42 | |||
| No chemotherapy | 28 (24/87) | 36 (24/67) | 0 (0/19) | |
| Neoadjuvant | 40 (35/87) | 40 (27/67) | 26 (5/19) | |
| Adjuvant | 32 (28/87) | 24 (16/67) | 74 (14/19) | |
| Location of primary tumor | ||||
| Colon | 33 (28/86) | 40 (24/67) | 42 (8/19) | |
| Sigmoid | 42 (36/86) | 28 (19/67) | 37 (7/19) | |
| Rectum | 26 (22/86) | 36 (24/67) | 21 (4/19) | |
| Latest type of preoperative imaging | 0.09 | |||
| CT | 70 (61/87) | 61 (41/67) | 100 (100/19) | |
| PET-CT | 7 (6/87) | 1 (1/67) | 0 (0/19) | |
| MRI | 13 (11/87) | 15 (10/67) | 0 (0/19) | |
| Primovist-enhanced MRI | 10 (9/87) | 22 (15/67) | 0 (0/19) | |
| Days between imaging and surgery, mean ± SD (n) | 34 ± 18 (87) | 38 ± 24 (67) | 0.24 | NA |
| ASA score | 0.52 | |||
| I | 12 (10/86) | 9 (6/67) | 0 (0/19) | |
| II | 80 (69/86) | 78 (52/67) | 53 (10/19) | |
| III | 8 (7/86) | 13 (9/67) | 47 (9/19) | |
| Type of surgery | 0.06 | |||
| Laparotomy | 94 (82/87) | 85 (57/67) | 100 (19/19) | |
| Laparoscopy | 5 (4/87) | 15 (10/67) | 0 (0/19) | |
| Conversion to laparotomy | 1 (1/87) | 0 (0/67) | 0 (0/19) | |
| Hemihepatectomy | 16 (14/87) | 10 (7/67) | 0.31 | 32 (6/19) |
| Radio Frequency Ablation (RFA) | 28 (24/87) | 24 (16/67) | 0.60 | 5 (1/19) |
| Node positive primary* | 51 (44/86) | 56 (36/64) | 0.54 | 68 (13/19) |
| Disease-free interval <12 months* | 76 (66/87) | 78 (52/67) | 0.80 | 68 (13/19) |
| More than 1 hepatic tumor* | 59 (51/87) | 49 (33/67) | 0.25 | 42 (8/19) |
| Largest hepatic tumor > 5cm* | 14 (12/87) | 9 (6/67) | 0.35 | 21 (4/19) |
| Preoperative CEA >200ng/ml* | 3 (2/72) | 2 (1/55) | 0.72 | 11 (2/19) |
| Synchronous metastases | 53 (46/87) | 43 (29/67) | 0.24 | 32 (6/19) |
| No. of CRLM on CT/MRI, median ; range (n) | 1 ;1 -5 (67) | 2 ;1 -7 (87) | 0.48 | 1 ;1 -4 (19) |
| Comorbidities | 0.25 | |||
| COPD | 5 (4/87) | 1 (1/67) | 26 (5/19) | |
| DVT or PE | 2 (2/87) | 3 (2/67) | 11 (2/19) | |
| CVE | 9 (8/87) | 1 (1/67) | 42 (8/19) | |
| DM | 10 (9/87) | 7 (5/67) | 74 (14/19) | |
| BMI, mean ± SD (n) | 26 ± 3.8 (85) | 26 ± 4.0 (67) | 0.80 | 25 ± 3.9 (19) |
NIRF: near-infrared fluorescence; LUMC: Leiden University Medical Center; SMGH: San Matteo General Hospital; SD: standard deviation; NA: not available; CRLM: colorectal liver metastases; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; DVT: deep venous thrombosis; PE: pulmonary embolism; CVE: cardiovascular event; BMI: body mass index.
Individual Fong criteria
Perioperative details (analysis 1)
The LUMC experimental cohort was compared with the control cohort. Results are shown in Table 2. No significant differences in volume of resected tissue, time of procedure and complications were seen.
Table 2.
Perioperative details (analysis 1)
| LUMC Control (n=87) |
LUMC Experimental (n=67) |
||
|---|---|---|---|
| mean ± SD (n) | mean ± SD (n) | p-value | |
| Total resected volume (cm3) / number of vital tumors resected | |||
| Other* | 62 ± 3.8 (66) | 57 ± 4.5 (54) | 0.76 |
| Hemihepatectomy* | 637 ± 2.4 (12) | 657 ± 2.7 (7) | 0.95 |
| Volume of healthy tissue resected per tumor (cm3)** | |||
| Other* | 26 ± 4.9 (128) | 24 ± 7.5 (110) | 0.79 |
| Hemihepatectomy* | 685 ± 2.2 (16) | 596 ± 1.9 (11) | 0.62 |
| Length of surgery (min) | 180 ± 53 (87) | 183 ± 56 (67) | 0.69 |
| Estimated blood loss (ml)* | 403 ± 2.8 (63) | 416 ± 3.3 (48) | 0.89 |
| Duration of hospital stay (days) - median ; range (n) | 6 ; 1 - 48 (87) | 6 ; 4 - 87 (67) | 0.99 |
| Reoperation - % (n/n) | 7 (6/87) | 7 (5/67) | 0.89 |
| 90-day mortality - % (n/n) | 0 (0/87) | 0 (0/67) | |
| Complications (Clavien-Dindo) - % (n/n) | 0.75 | ||
| Grade III | 8 (7/87) | 7 (5/67) | |
| Grade IV | 5 (4/87) | 7 (5/67) |
Logarithmic transformation was used in the analysis of the data
For each resected tumor (vital or non-vital) the spherical volume of the tumor diameter was subtracted from the estimated specimen volume.
Tumor characteristics (analysis 1)
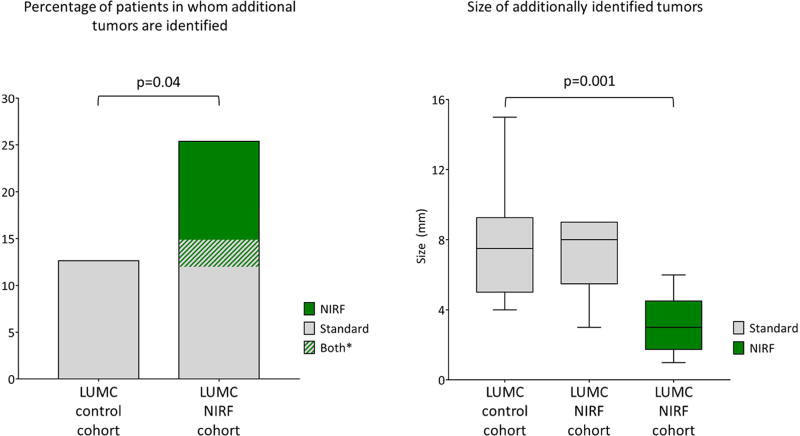
Tumors resected in the LUMC experimental cohort were compared with those in the control cohort (Figure 3). The median number of preoperatively identified metastases did not differ significantly (1 [range 1–5] vs. 2 [1–7], respectively; p=0.48). The median number of resected or ablated tumors also did not differ significantly (2 [1–10] vs. 2 [1–7]; p=0.81). However, the percentage of patients in whom additional lesions were identified during surgery was significantly higher in the LUMC experimental cohort (25% vs. 13%; p=0.04). The difference was the result of lesions identified by NIRF only; there was no significant difference when using standard techniques only (15% vs. 13%; p=0.68). In 3% (n=2) additional metastases were identified by standard techniques, but also other metastases by NIRF imaging only. The mean size of additional tumors identified by NIRF imaging only was significantly smaller than the size of tumors identified by standard techniques (3.2 ± 1.8 mm vs. 7.4 ± 2.6 mm; p<0.001). Tumors in the LUMC experimental cohort were resected radically (R0 resection) in 83% of cases vs. 79% of tumors in the control cohort (p=0.41). Overall, sensitivity of NIRF imaging was 83%. Sensitivity of only superficial (i.e. < 8 mm subcapsular) CRLM was 100%. Intraoperative identification of additional CRLM was not significantly correlated with the interval between last preoperative imaging and surgery.
Figure 3. Additionally identified colorectal liver metastases (analysis 1).
Additionally identified colorectal liver metastases. The percentage of patients in whom additional metastases were identified during surgery was significantly higher in the LUMC experimental cohort compared to the LUMC control cohort. Tumors identified solely by near-infrared fluorescence imaging were significantly smaller compared to additional metastases identified using standard techniques.
* In 3% (n=2) additional metastases were identified with standard techniques, but NIRF imaging identified even more unknown metastases.
Long-term follow-up results (analysis 1)
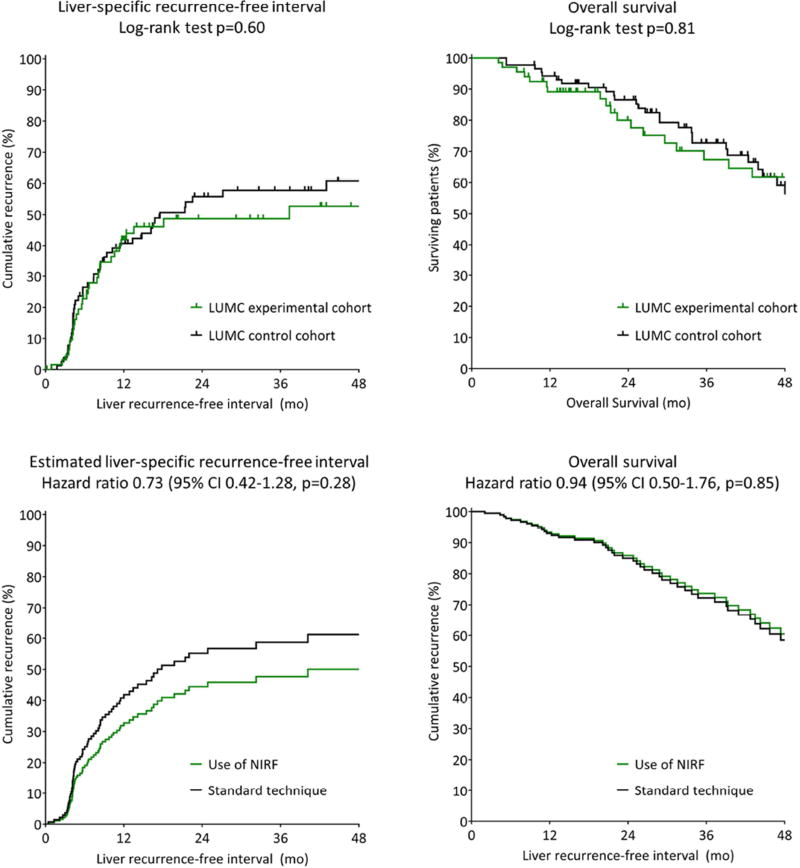
The follow-up of the LUMC experimental cohort was compared with the control cohort (Figure 4). Median follow-up was 46 (range 4–80) and 43 (5–79) months, respectively. In the experimental cohort 47% did not have a recurrence in the liver at 4 years follow-up, compared to 39% in the control cohort (p=0.40). Overall survival at 4 years was 62% and 59%, respectively (p=0.79). A Cox proportional hazards regression analysis was performed, including use of NIRF imaging, age, gender, chemotherapy, ASA score, surgery type, and Fong’s clinical risk score (0–4; CEA>200 ng/ml was excluded due to a high number of missing values). The hazard ratio for liverspecific recurrence when using NIRF imaging was 0.73 (95% confidence interval [CI] 0.42–1.28; p=0.28). The hazard ratio for overall survival was 0.94 (95% CI 0.50–1.76, p=0.85).
Figure 4. Liver-specific recurrence-free interval and overall survival (analysis 1).
(Estimated) liver-specific recurrence-free interval and overall survival. LUMC: Leiden University Medical Center; SMGH: San Matteo General Hospital; NIRF: near-infrared fluorescence.
Survival after resection of metastases identified solely by NIRF imaging (analysis 2)
Additional subcapsular CRLM were visualized by fluorescence imaging only in 24% (21/86) of patients who underwent NIRF-guided resection of CRLM either at the LUMC (n=9) or the SMGH (n=12). At 3 years follow-up 52% of those patients did not have recurrent disease in the liver; 48% did not have any recurrence at all.
DISCUSSION
The only option to cure patients with CRLM is a complete resection of all lesions. Yet, intrahepatic recurrence rates after surgery using only white light remain high.[1–3] One of the main problems is the fact that subcentimeter lesions cannot be detected easily, neither before nor during surgery.[5–7, 17] Even with state-of-the-art scanners and up-to-date protocols, metastases are still being missed, as experienced in this study. Moreover, metastases may arise and/or grow in the period between preoperative imaging and surgery. Novel techniques for better intraoperative distinction between malignant and benign tissue are urgently needed. This is the first study that presents long-term follow-up after NIRF-guided resection of CRLM, although the non-randomized design limits its level of evidence. NIRF imaging of CRLM using ICG was safe and time-efficient, while significantly more and smaller tumors were identified during surgery. Removal of metastases solely identified by NIRF appears helpful; 52% of patients did not develop liver recurrence after resection of vital tumor cells. These additional metastases would – if not resected or treated by chemotherapy – almost certainly result in recurrence of liver metastases. In the Netherlands, neoadjuvant chemotherapy is not standard-of-care, since there is no clear survival benefit, while there are considerable side-effects.[25]
At the LUMC additional tumors were identified in 13% of all patients. Assuming that 52% of these patients (i.e. 7%) do not develop a recurrence, the number-needed-to-treat would be 14. ICG is inexpensive; a vial of 25 mg costs approximately $85 USD. Leaving the costs of purchasing a fluorescence imaging system aside, 14 doses of ICG cost $1190 USD, while treating a recurrence of CRLM will cost a plurality. Several NIRF imaging systems are already available for clinical use.[26]
This study failed however to show substantial evidence that the addition of NIRF imaging positively influences clinical outcome measures including recurrence-free interval and OS. A study powered to show a significant difference of 7% would require a total of >1,500 patients. This means that the current study is severely underpowered to show such a difference.
Although significantly more tumors were identified in the LUMC experimental cohort, patients still developed recurrences, of which the majority within the first year. This means that tumors were being missed by NIRF imaging, potentially due to the fact that not the entire liver surface was interrogated. During surgery it proved to be difficult to reach the posterior segments of the liver due to either the size of the camera head or because the liver was not always fully mobilized off the diaphragm. A smaller, hand-held camera head, or the combined use of open and minimally-invasive imaging systems, could increase the percentage of the liver surface that can be imaged. Furthermore, although tumors additionally identified by NIRF imaging are significantly smaller, submillimeter tumors might still be missed. Finally, tumors at a depth of more than 8 mm from the liver surface are currently not identifiable by NIRF imaging.[12] The technique can therefore only be applied in combination with IOUS, as done in this study.
No significant difference was shown in radical resection rates of both LUMC cohorts. Especially during open surgery, the fluorescence imaging systems were mostly used during screening of the liver surface and not during resection. Of note, no attempt was made to compare the performance among imaging systems, and it is possible that variation in detectability exist. All open space imaging systems, however, provided surgeons simultaneously with real-time color, NIRF and color-NIR merge images. The Storz HD laparoscope only had a color and NIRF modus. Switching was done using a foot pedal.
ICG can also be applied in combination with 5-ALA for the detection of CRLM.[14] Although combined use improved specificity, 5-ALA resulted in adverse events, including vomiting and serum AST elevation, in 14% of all patients. Furthermore, the emitted fluorescence of 5-ALA (peak at 635 nm) is subject to higher absorbance and penetrates only approximately 2 mm tissue. NIRF imaging with ICG for the detection of superficial CRLM is sensitive, but cannot detect extrahepatic metastases. Conjugation of fluorophores to targeting moieties, such as antibodies, can enable detection of all tumor lesions, regardless of location. This method was explored in several clinical trials, including breast, ovarian and head and neck cancer patients.[27–29] For CRLM a clinical trial with SGM-101, which targets CEA is currently ongoing (Netherlands Trial Register number NTR5673). Unfortunately, clinical translation of targeted fluorescent tracers is expensive and time-consuming and requires specific expertise. Until hurdles associated with clinical translation of novel tracers are overcome, ICG can be used as a safe, inexpensive and clinically available alternative. Moreover, as SGM-101 is fluorescent at approximately 700 nm, it could be simultaneously applied with ICG. Several NIRF imaging systems, including the Artemis and FLARE® systems, can perform dualwavelength imaging.
CONCLUSION
In conclusion, this study suggests that NIRF imaging during resection of CRLM identifies significantly more and smaller tumors, preventing recurrences in a subset of patients. Given its safety profile and low expense, routine use can be considered until tumor targeting fluorescent tracers are available for clinical use.
Acknowledgments
This work was supported by the Dutch Cancer Society (grant UL2010-4732 and UL2012-5561) as well as NIH grants R01-CA-185457 and R01-DE-022820. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We thank Gerrit-Jan Liefers, Volkert Huurman, and David Lam for their assistance and clinical expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: John V. Frangioni is currently CEO of Curadel, Curadel ResVet Imaging, and Curadel Surgical Innovations, which are for-profit companies that have licensed FLARE® technology from Beth Israel Deaconess Medical Center. All other authors have no conflicts of interests or financial ties to disclose.
References
- 1.Wei AC, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668–76. doi: 10.1245/ASO.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Abdalla EK, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25. doi: 10.1097/01.sla.0000128305.90650.71. discussion 25-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees M, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–35. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 4.Karanjia ND, et al. Survival and recurrence after neo-adjuvant chemotherapy and liver resection for colorectal metastases: a ten year study. Eur J Surg Oncol. 2009;35:838–43. doi: 10.1016/j.ejso.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Frankel TL, et al. Preoperative imaging for hepatic resection of colorectal cancer metastasis. J Gastrointest Oncol. 2012;3:11–8. doi: 10.3978/j.issn.2078-6891.2012.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalil H, et al. Hepatic lesions deemed too small to characterize at CT: prevalence and importance in women with breast cancer. Radiology. 2005;235:872–8. doi: 10.1148/radiol.2353041099. [DOI] [PubMed] [Google Scholar]
- 7.Holzapfel K, et al. Comparison of diffusion-weighted MR imaging and multidetector-row CT in the detection of liver metastases in patients operated for pancreatic cancer. Abdom Imaging. 2011;36:179–84. doi: 10.1007/s00261-010-9633-5. [DOI] [PubMed] [Google Scholar]
- 8.Vahrmeijer AL, et al. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–18. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stummer W, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 10.Stummer W, et al. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93:1003–13. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 11.Stummer W, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid- induced porphyrin fluorescence. Neurosurgery. 1998;42:518–25. doi: 10.1097/00006123-199803000-00017. discussion 25-6. [DOI] [PubMed] [Google Scholar]
- 12.Ishizawa T, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–504. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]
- 13.Ishizuka M, et al. Intraoperative observation using a fluorescence imaging instrument during hepatic resection for liver metastasis from colorectal cancer. Hepatogastroenterology. 2012;59:90–2. doi: 10.5754/hge11223. [DOI] [PubMed] [Google Scholar]
- 14.Kaibori M, et al. Intraoperative Detection of Superficial Liver Tumors by Fluorescence Imaging Using Indocyanine Green and 5-aminolevulinic Acid. Anticancer Res. 2016;36:1841–9. [PubMed] [Google Scholar]
- 15.van der Vorst JR, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer. 2013;119:3411–8. doi: 10.1002/cncr.28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peloso A, et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB (Oxford) 2013;15:928–34. doi: 10.1111/hpb.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boogerd LS, et al. Laparoscopic detection and resection of occult liver tumors of multiple cancer types using real-time near-infrared fluorescence guidance. Surgical endoscopy. 2016 doi: 10.1007/s00464-016-5007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barabino G, et al. Improving Surgical Resection of Metastatic Liver Tumors With Near- Infrared Optical-Guided Fluorescence Imaging. Surgical innovation. 2016;23:354–9. doi: 10.1177/1553350615618287. [DOI] [PubMed] [Google Scholar]
- 19.Benya R, et al. Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn. 1989;17:231–3. doi: 10.1002/ccd.1810170410. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal EL, et al. Successful Translation of Fluorescence Navigation During Oncologic Surgery: A Consensus Report. J Nucl Med. 2016;57:144–50. doi: 10.2967/jnumed.115.158915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong Y, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. discussion 18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troyan SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first- in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–52. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Driel PB, et al. Characterization and evaluation of the artemis camera for fluorescence- guided cancer surgery. Mol Imaging Biol. 2015;17:413–23. doi: 10.1007/s11307-014-0799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boogerd LS, et al. Application of near-infrared fluorescence imaging during modified associating liver partition and portal vein ligation for staged hepatectomy. Surgery. 2016;159:1481–2. doi: 10.1016/j.surg.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Nordlinger B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 26.Handgraaf HJ, et al. Real-time near-infrared fluorescence guided surgery in gynecologic oncology: a review of the current state of the art. Gynecol Oncol. 2014;135:606–13. doi: 10.1016/j.ygyno.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Hoogstins CE, et al. A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clin Cancer Res. 2016;22:2929–38. doi: 10.1158/1078-0432.CCR-15-2640. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal EL, et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res. 2015;21:3658–66. doi: 10.1158/1078-0432.CCR-14-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamberts LE, et al. Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0437. [DOI] [PubMed] [Google Scholar]