Figure 4.
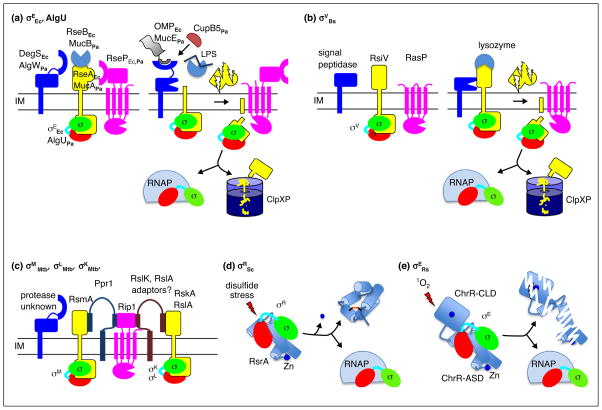
Envelope sensing by regulated proteolysis and redox sensing by conformational changes. (a) Roles of the key players in the pathways regulating σEEc and AlgU are depicted as the system transitions from the inactive to the active state. Proteolysis of RseA and MucA is triggered by binding of OMP peptides to DegS and AlgW in conjunction with release RseB and MucB from the anti-sigma factor upon LPS binding. For P. aeruginosa, CupB5 can activate AlgW bypassing the need for LPS binding to MucB. (b) Activation of σV by degradation of RsiV following lysozyme binding is depicted. In the inactive state, the first protease to act, signal peptidase, cannot access RsiV. Binding of lysozyme to RsiV exposes the cleavage site in RsiV to signal peptidase initiating the proteolytic cascade. (c) A related proteolytic pathway regulates degradation of the RsmA, RskA, and RslA anti-sigma factors from M. tuberculosis. The initiating protease and inducing signals have yet to be identified. Rip1 is responsible for the second cleavage in the proteolytic cascade for all three anti-sigma factors. The adapter protein, Ppr1, bridges Rip1 and RsmA and prevents cleavage. Similar adapter proteins have been postulated for RskA and RslA. (d) Redoxing sensing by RsrA is depicted. In the inactive state, σR is sequestered by the reduced Zn bound form of RsrA. Exposure to reactive electrophile species results in loss of Zn, formation of an intramolecular disulfide bond, structural rearrangement of RsrA, and release of σR. (e) Activation of σE by singlet oxygen (1O2). In the inactive state, σE is bound to ChrR. Singlet oxygen sensing involves ligands of the Zn atom in the ChrR-CLD, although loss of Zn is not absolutely required, and leads to degradation of ChrR by an unknown mechanism.