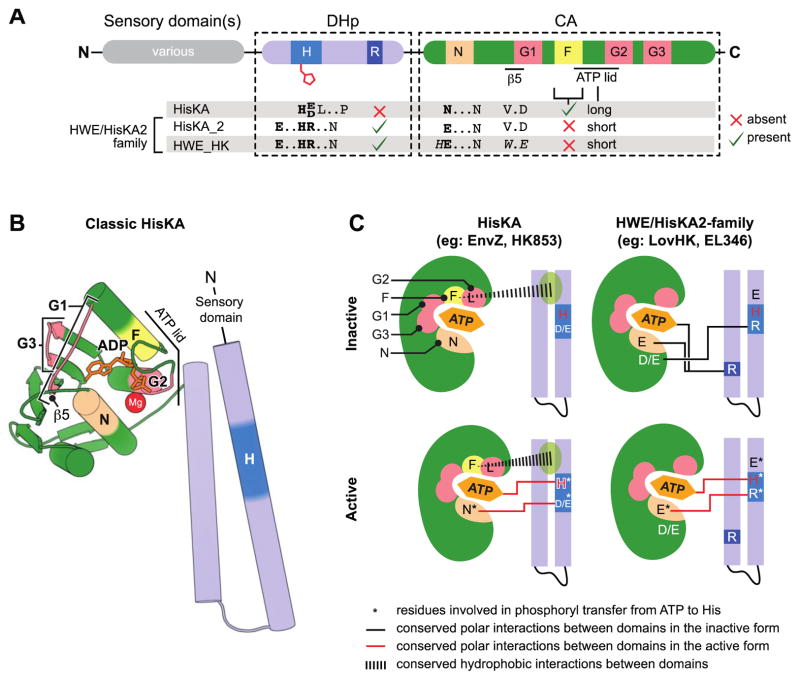
Figure 1. Characteristic features which distinguish HWE/HisKA2 histidine kinases.
(A) Primary structure features. Domain organization (top) highlights conserved histidine kinase boxes (H, R, N, G1, F, G2, G3). Distinguishing sequence motifs in Pfam:HisKA, Pfam:HisKA_2 or Pfam:HWE_HK kinases are highlighted below the domains. HisKA2 and HWE_HKs motifs are closely related. (B) Structural organization of a classical HisKA-family histidine kinase based on the crystal structure of Thermotoga maritima HK853 (PDB: 2C2A)[24]. The DHp domain (blue) forms two alpha helices that mediate dimerization and contain the phosphorylated histidine (dark blue). The catalytic ATPase (CA) domain (green) adopts the same basic globular fold as other GHKL ATPase superfamily domains [54]. Highlighted regions correspond to conserved boxes colored as in panel A. Sensory input domains are N-terminal to the DHp domain. (C) Conserved interdomain interactions in active and inactive conformations in HisKA and HWE/HisKA2 kinases. The CA domain is cartooned as a shell around the ATP binding pocket. The approximate positions of the conserved boxes are colored as in A and B. Key regulatory residues are marked with letters within the structure models. Residues involved in catalyzing histidine phosphorylation are highlighted with an asterisk. Conserved polar interactions between DHp and CA domains are shown as black lines in the inactive conformations and red lines in the active conformations.