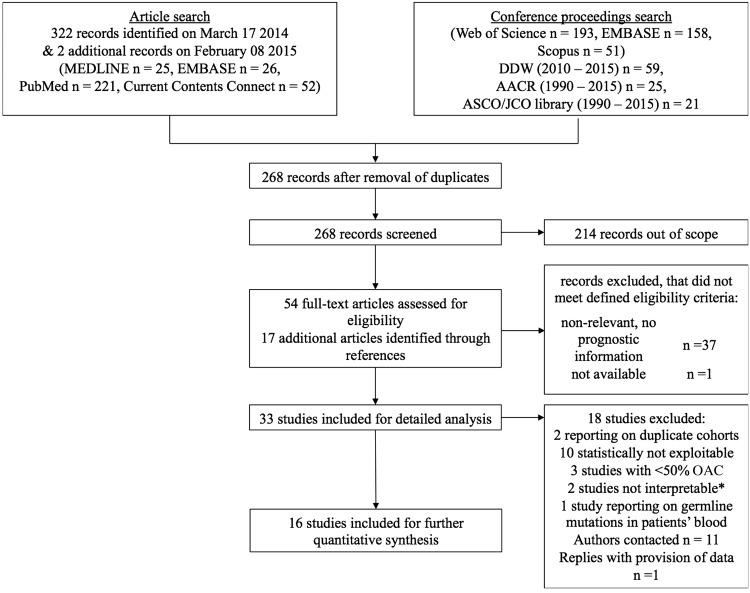
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of study identification process. *One study provided sufficient information for statistical extraction; however, interpretation of data was not possible due to inconsistent definition of protein 53 (p53) mutation status in survival analysis, in as much that p53 positive denoted a change from p53 positivity pre-radiochemotherapy to p53 negative post-chemoradiotherapy. One study only presented final survival data on loss of heterozygosity at chromosome 17q, which does not contain the tumour protein 53 (TP53) gene. AACR, American Association for Cancer Research; ASCO, American Society for Clinical Oncology; DDW, Digestive Disease Week; JCO, Journal of Clinical Oncology.