Abstract
An 81-year-old man from rural Australia presented with right pretibial cellulitis 7 days after minor trauma against furniture. He failed to improve despite antibiotics and surgical debridement. Subsequent cultures grew the rare fungus Saksenaea vasiformis, which was treated with further surgical debridement, amphotericin B and posaconazole. This was successful and the patient made a full recovery. We present the case and discuss lessons learnt.
Keywords: infectious diseases, general surgery, drugs: infectious diseases
Background
Saksenaea vasiformis is a rare fungal pathogen in humans, with less than 50 documented cases. It can cause severe soft tissue and systemic infections. Prompt antimicrobial therapy supported by debridement when necessary leads to a favourable outcome in most cases, especially in localised subcutaneous infections. Disseminated infection is almost uniformly fatal. Laboratory identification of this fungus is crucial but challenging. Instances of cellulitis that fail either to improve with typical therapy or to grow causative bacteria should arouse clinical suspicion of atypical organisms such as fungi.
Case presentation
An 81-year-old man attended his regional hospital with bilateral pretibial injuries, sustained 9 days earlier while moving furniture at his home in rural Australia. He stated his pain had increased following playing golf 2 days prior to presentation, but denied bleeding, difficulty weight bearing nor systemic symptoms. He had a medical history of atrial fibrillation, hypertension and hyperlipidaemia. His medications consisted of warfarin 3 mg daily, and morning amiodarone 100 mg, pantoprazole 40 mg and perindopril 5 mg. Examination revealed superficial lacerations on both anterior lower limbs. The right wound was also tender and erythematous but without fluctuance nor purulent discharge to suggest abscess. The contralateral wound was healing well. The patient was afebrile with normal heart rate and blood pressure. The patient was admitted to the hospital and was given intravenous flucloxacillin 2 g four times per day, with daily Mepitel® wound dressings. Initial serum investigations revealed haemoglobin of 119 g/L, white cell count of 9.97×109/L, platelets of 204×109/L, estimated glomerular filtration rate of 28 mL/min/1.73 m2 and a C reactive protein of 210 mg/L.
His pain progressed despite the antibiotic therapy, and 5 days later (14 days postinjury) the right pretibial erythema had broken down into a 5×5 cm wound with surrounding erythema and oedema. His wound initially appeared to improve, then redeteriorated. 24 days postinjury, his wound had enlarged to 8×8 cm with central slough and a distinct margin, and developed malodour, purpura and fluctuance, suggestive of an underlying haematoma (figure 1).
Figure 1.
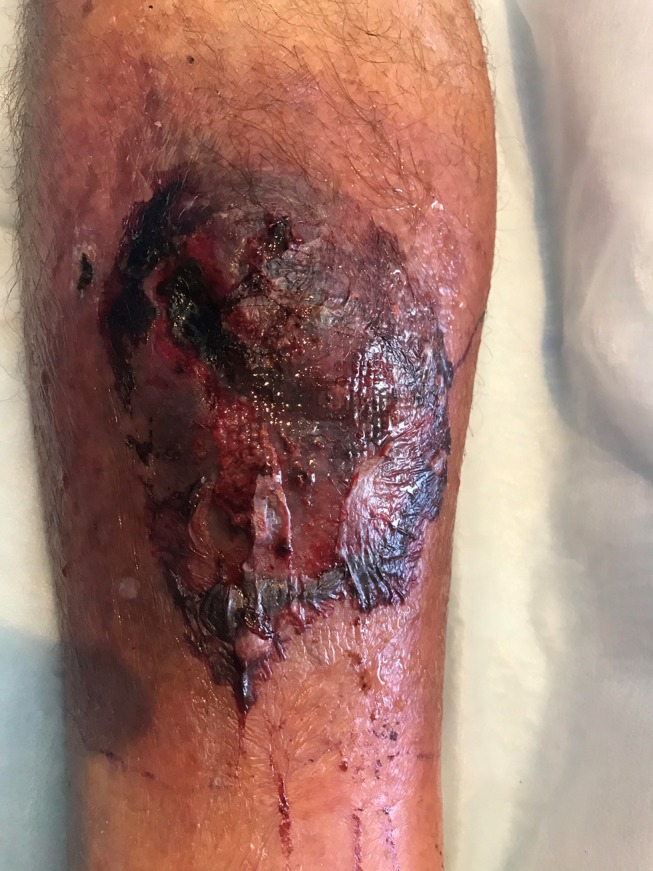
Anterior right lower limb wound day 18 postinjury. The 8×8 cm well-demarcated wound displays central slough, with surrounding fluctuance and purpura, suggesting deeper haematoma.
After escalating antibiotic therapy to intravenous vancomycin 2 g daily and intravenous piperacillin/tazobactam 4 g/500 mg four times per day, the patient was taken to theatre the same day for surgical debridement. Intraoperatively, a 10×9 cm area of soft tissue was found to be non-viable. This was resected and a vacuum dressing applied.
The next morning the wound was satisfactory. However, the following day (26 days since the initial injury), the wound had deteriorated significantly, with extension of the wound and spreading cellulitis (figure 2).
Figure 2.
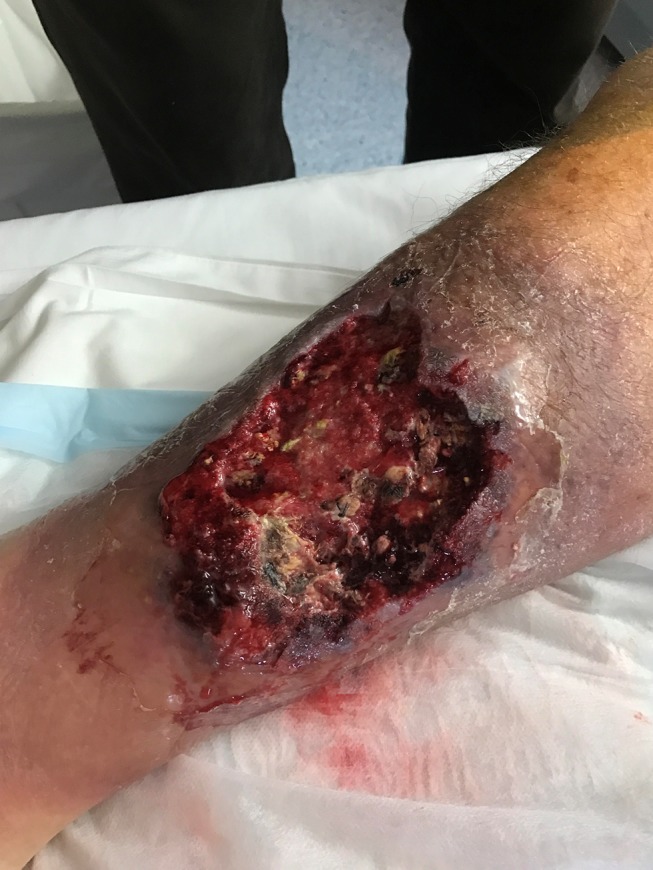
Two days post first debridement (day 26 post injury), the wound has deteriorated, with an irregular, advancing edge and raw ulcerated centre.
Investigations
Wound swabs from day of admission initially grew multisensitive Staphylococcus aureus. Plain films of the limb displayed soft tissue swelling and no subcutaneous gas, foreign body nor bony abnormality. Blood cultures through the patient’s course were consistently negative.
The failure to improve prompted investigation of vascular compromise. Duplex ultrasound showed no features of venous incompetence. Arterial ultrasound similarly did not reveal a cause.
Differential diagnosis
The patient was initially thought to have cellulitis due to S. aureus. Poor response raised suspicion of an atypical pathogen, such as fungi.
Treatment
Day 26 postinjury, simultaneous with observation of wound progression, intraoperative swabs returned strongly positive for a fungal infection. The patient was transferred the following day to a metropolitan tertiary centre for urgent input from specialty infectious diseases and plastic and reconstructive surgical departments.
The patient was given intravenous amphotericin B liposomal 160 mg as a 24-hour infusion, and continued on the intravenous vancomycin and piperacillin/tazobactam. Unfortunately the patient appeared to sustain a reaction to the amphotericin B, experiencing acute central chest and back pain, dyspnoea and tachypnoea. An ECG revealed non-specific changes, serial serum troponins were not elevated and echocardiogram was normal.
The amphotericin B was held for 3 days, then cautiously uptitrated to 400 mg daily. The patient did not suffer any further adverse symptoms, although required near-daily intravenous replacement for hypokalaemia and hypomagnesaemia. His admission was further complicated by acute kidney injury and fluid overload, which resolved with conservative management. He underwent further wound debridement on days 31 and 34 postinjury, with a split skin graft applied on the latter date from a donor site on the right upper thigh.
At this point, culture from the initial debridement using tap water agar media cultured S. vasiformis (figure 3). In consultation with the infectious diseases department, amphotericin B was ceased and the patient was given oral posaconazole 300 mg controlled-release daily. His amiodarone was ceased, given the known interaction between these medications.
Figure 3.
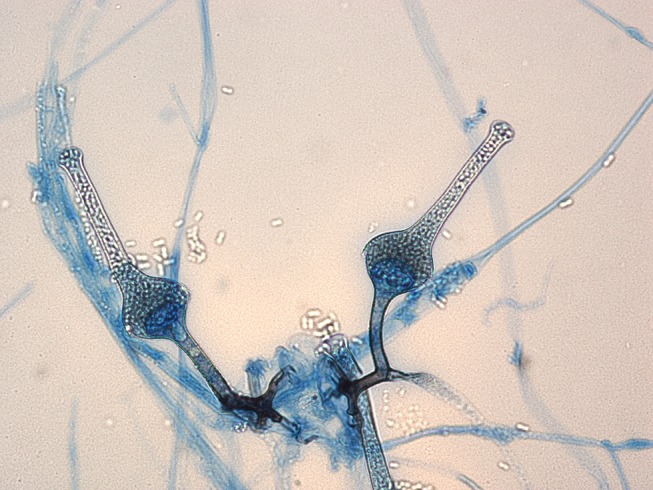
Microscopy of fungi grown from this patient. Images provided courtesy of pathologists Dr Sarah Kidd and Dr Helen Alexiou, National Mycology Reference Centre within the South Australian Pathology Centre, Adelaide.
Outcome and follow-up
He remained on posaconazole controlled-release 300 mg daily, which will continue for a total of 3 months. Follow-up wound and blood cultures were negative, and the wound steadily improved.
The patient had become deconditioned by his prolonged admission, but soon was able to mobilise independently through regular physiotherapy. On day 50 postinjury, he was transferred back to the regional hospital nearer his home for ongoing rehabilitation. His wound looked excellent (figure 4) and continued to improve.
Figure 4.
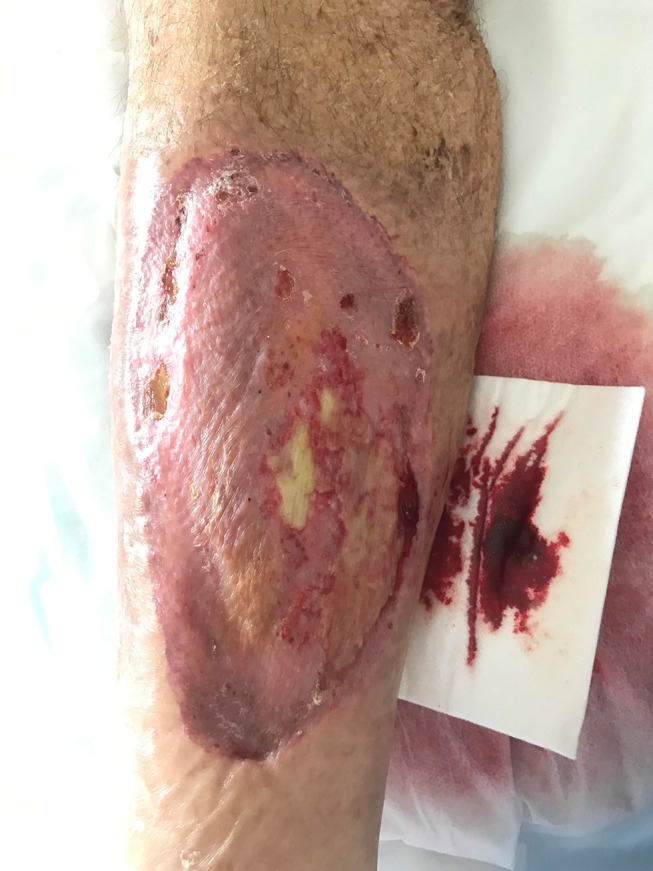
Right lower limb wound day 54 postinjury, with progressive successful take of the split skin graft.
Unfortunately his discharge was delayed due to Clostridium difficile colitis. On day 62 postinjury, he was retransferred to the metropolitan tertiary centre, receiving metronidazole oral 400 mg three times per day and physiotherapy. At time of writing, 3 months postinjury, he remains well and continuing to rehabilitate towards intended discharge home.
Discussion
S. vasiformis belongs to class Zygomycetes and order Mucorales. It was first described in 1953 by Dr SB Saksena, who isolated this fungus from forest soil in India. Subsequently, Saksenaea species have been found worldwide. Like all Mucorales fungi, they are ubiquitous in nature. Infection typically results from the traumatic implantation of soil or other organic material containing sporangiospores. Human infections have been documented in North, Central and South America, and in Europe, Asia and Australia.1 However, these have been rare, with less than 50 cases reported since its discovery.2
The first human infection by S. vasiformis was reported in 1976 in the facial wounds of a 19-year-old man following an automobile accident.3 Such a presentation has become the norm. Most patients are immunocompetent and suffer infections of open wounds. Injury mechanism affects risk of skin inoculation, with trauma types including surgery, burns, motor vehicle accidents, intravenous needle use and insect or spider bite. Infection through inhalation has also been reported.4 Immunocompromise is a distinct risk factor.5
S. vasiformis typically causes localised cutaneous and subcutaneous infections. These are usually treatment-responsive and run a benign course.2 Early detection is key, as disseminating disease is nearly uniformly fatal.1
Like other Mucorales, S. vasiformis has broad irregularly branching hyphae with rare septations. Despite its classical shape, diagnosis is challenging. S. vasiformis grows easily on routine media, producing white hyphae, like other zygomycetes.6 However, these hyphae are sterile, and on typical media do not produce any identifying sporulating structures. Laboratories must use nutrient-deplete media such as tap water agar to induce sporulation. In this environment, the fungus needs to sporulate to survive, and within 1–2 weeks these distinct structures will develop and allow microscopic visualisation. In the case of Saksenaea, its distinctive funnel-shaped, pigmented sporangia develop sporangiospores from their apex, which are pathognomonic.
This challenge of microscopic identification delays diagnosis. Hence, clinicians should be suspicious when traumatic wounds fail to improve with apparently appropriate therapy, and seek out atypical organisms such as S. vasiformis. This may allow belated but crucial therapy for patients with spreading infection, as with this case.
Treatment consists of debridement and antifungal therapy. Traditionally amphotericin B has been the mainstay of treatment. However, it may cause nephrotoxicity. An alternative is the new-generation triazole antifungal posaconazole. This may avoid these side effects or be used as salvage therapy when amphotericin B has failed.
Patient’s perspective.
“It all started when I tripped and hit both my shins in the yard. The right wound just blew out within the week. First they treated me for a bacterial infection and then the next thing I know, I was transferred to the bigger hospital to treat this fungal infection. They patched me up with some skin off my thigh and I was transferred home a month later. It was difficult being away from my wife who was unwell, and it seemed to take a long time to work out what was wrong with my leg.”
Learning points.
Saksenaea vasiformis is a highly uncommon human pathogen that may not be well known to many clinical laboratories.
Suspicion should be raised in patients with wounds which deteriorate in the face of usual management.
Culture is necessary for diagnosis but challenging, requiring specialised media.
Management consists of antimicrobials amphotericin B and azoles, and aggressive surgical debridement when indicated.
Acknowledgments
The authors were grateful for the generous assistance of pathologists Dr Sarah Kidd and Dr Helen Alexiou, National Mycology Reference Centre within the South Australian Pathology Centre, Adelaide.
Footnotes
Contributors: WTP was responsible for manuscript concept and initial manuscript. NK and DBH revised the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kaushik R, Chander J, Gupta S, et al. Fatal primary cutaneous zygomycosis caused by Saksenaea vasiformis: case report and review of literature. Surg Infect 2012;13:125–9. 10.1089/sur.2010.078 [DOI] [PubMed] [Google Scholar]
- 2.Gomes MZ, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev 2011;24:411–45. 10.1128/CMR.00056-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajello L, Dean DF, Irwin RS. The zygomycete Saksenaea vasiformis as a pathogen of humans with a critical review of the etiology of zygomycosis. Mycologia 1976;68:52–62. 10.2307/3758897 [DOI] [PubMed] [Google Scholar]
- 4.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000;13:236–301. 10.1128/CMR.13.2.236-301.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam RD, Hunter G, DiTomasso J, et al. Mucormycosis: emerging prominence of cutaneous infections. Clin Infect Dis 1994;19:67–76. 10.1093/clinids/19.1.67 [DOI] [PubMed] [Google Scholar]
- 6.Vega W, Orellana M, Zaror L, et al. Saksenaea vasiformis infections: case report and literature review. Mycopathologia 2006;162:289–94. 10.1007/s11046-006-0061-6 [DOI] [PubMed] [Google Scholar]


