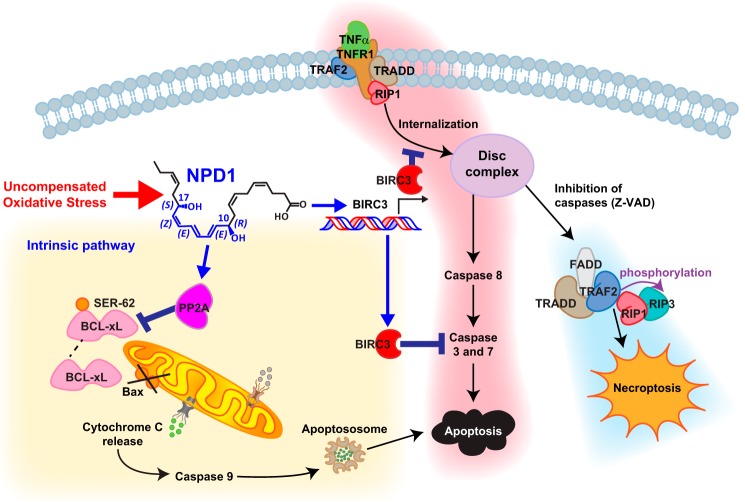
Figure 1.
NPD1 inhibits apoptosis during UOS. Ligation of TNFR1 (highlighted in pink) stimulates the formation of complex I, which consists of TNFR1, TRADD, RIPK1, TNFR-associated factor 2 (TRAF2), BIRC2, and BIRC3. In the absence of BIRCs, RIP1 is deubiquitinated, which results in internalization of complex I, and procaspase-8 and RIP3 are integrated to form a new signaling complex (complex II or DISC). BIRCs suppress the formation of this complex, which acts as the activation platform for caspase-8, which induces cell death by the extrinsic pathway. Under conditions in which caspase activation is inhibited, RIP1 and RIP3 may be phosphorylated, thus forming a complex named necroptosome, which leads to cell death through a caspase-independent process known as necroptosis (highlighted in blue). UOS-triggered NPD1 exerts anti-apoptotic activity by increasing BIRC3 expression, resulting in subsequent augmentation of its anti-apoptotic effects. NPD1 also promotes dephosphorylation of Bcl-xL and promotes its heterodimerization with pro-apoptotic Bax, resulting in the consequent inactivation of this protein (highlighted in yellow). Z-VAD, benzyloxycarbonyl-VAD.