Figure 2.
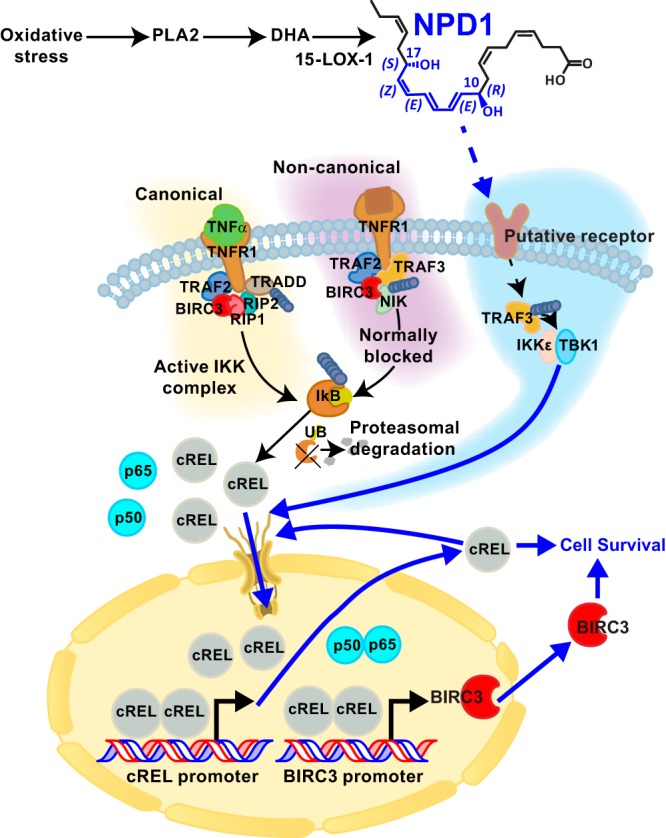
Up-regulation of BIRC3 transcription by NPD1-dependent c-REL expression. Stimulus-driven proteosomal degradation of IκB proteins is mediated by the IKK complex, which liberates NF-κB dimers and results in their further translocation into the nucleus and transcriptional activity. NF-κB signaling often is divided into two types of pathways. The canonical pathway (yellow) is induced by most physiological NF-κB stimuli and is represented here by TNFR1 signaling in an IKKβ- and NEMO-dependent manner, with resulting activation of RelA containing heterodimers. In contrast, the non-canonical pathway (purple) is stimulated by certain TNF family cytokines, such as CD40L and lymphotoxin-β, and it involves IKKα-mediated phosphorylation and generation of p52-RELB complexes in a NIK-dependent manner, which is subject to a complex regulation by TRAF3, BIRC3, and other ubiquitin (UB) ligases. Activation of both pathways could generate c-REL containing hetero- or homodimers. NPD1, which is synthesized in response to oxidative stress in the eye and brain, triggers the production of c-REL-containing dimers independent of both the canonical and non-canonical NF-κB pathways, possibly by modifying the c-REL TBK1/IKKϵ axis via activation of a putative receptor (blue).
