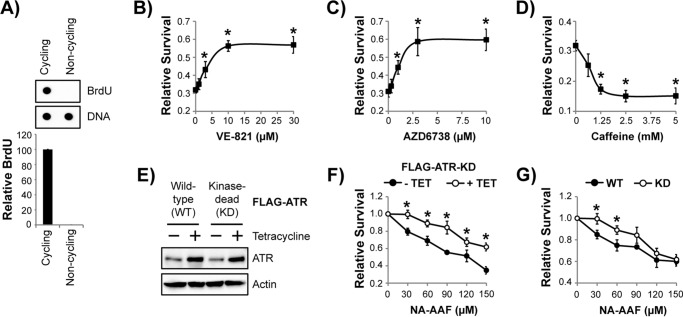
Figure 1.
Pharmacological and genetic inhibition of ATR kinase protects non-replicating cells from the lethal effects of the UV mimetic NA-AAF. A, cycling and non-cycling HaCaT cells were pulsed with 10 μg/ml BrdU for 15 min. Genomic DNA was then purified and analyzed by immunodot blotting with the indicated antibodies. The graph shows the relative level of BrdU incorporation into genomic DNA (normalized to cycling cells) from three independent experiments. B, non-cycling HaCaT cells were treated with the indicated concentration of the ATR inhibitor VE-821 for 30 min prior to treatment with 15 μm NA-AAF. Cells were stained with crystal violet 24 h later to determine relative survival. C, cells were treated with the indicated concentration of AZD6738 and analyzed as described in B. D, cells were treated with caffeine and analyzed as described in B. E, non-cycling U2OS cells containing either a WT or KD FLAG-tagged ATR transgene under the control of a tetracycline-inducible promoter were left untreated or treated with 1 μg/ml tetracycline for 48 h before analysis by immunoblotting. F, non-cycling U2OS cells containing the FLAG-ATR-KD transgene were left untreated (− TET, no tetracycline) or treated with tetracycline (+ TET) for 48 h before exposure to the indicated concentration of NA-AAF. After an additional 48 h, cells were stained with crystal violet to determine relative survival. G, non-cycling U2OS cells induced to express the indicated form of ATR were treated with NA-AAF as in E to determine relative cell survival. *, p < 0.05; indicating a significant difference in survival between the two treatments or cell lines.