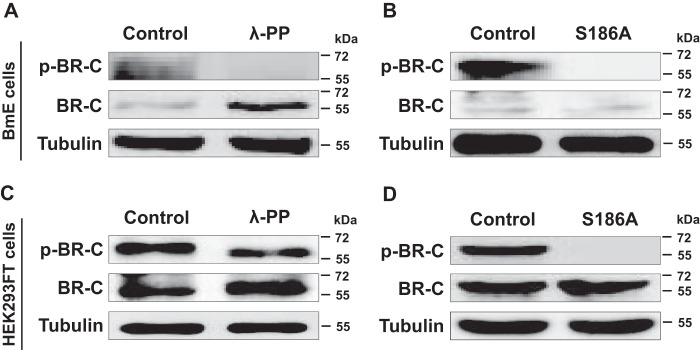
Figure 2.
Ser-186 of BR-C was confirmed in vitro as a phosphorylated site. A, changes in the phosphorylation of silkworm BR-C protein after λ-PP treatment in silkworm BmE cells. The protein extracted from BmE cells overexpressing BR-C was treated with λ-PP for 0.5 h and subsequently assayed via Western blotting using anti-BR-C and anti-p-BR-C antibodies. B, changes in the phosphorylation of silkworm BR-C protein after S186A mutation in BmE cells. BR-C with the S186A mutation was overexpressed in the BmE cells for 48 h, and the extracted total proteins were used in Western-blotting experiments. C, phosphorylation changes of silkworm BR-C protein after λ-PP treatment of human HEK293FT cells overexpressing intact BR-C. D, phosphorylation change of silkworm BR-C protein with the S186A mutation in human HEK293FT cells.