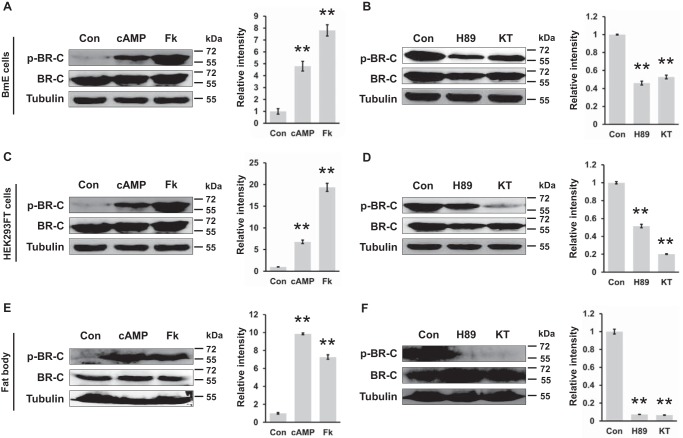
Figure 3.
Ser-186 of BR-C was characterized as a PKA phosphorylation site. A, at 48 h after transfection with the overexpression vector pSL1180-BR-C, the BmE cells were treated with 160 nm cAMP or 20 μm forskolin for 12 h; Fk, forskolin. DMSO treatment was used as a control (Con). B, inhibition of PKA activity by H89 or KT5720 treatment attenuated phosphorylation of BR-C at Ser-186. The BR-C-overexpressing BmE cells were treated with 20 μm H89 or 100 nm KT5720 for 4 h; KT, KT5720. C, activation of PKA in HEK293FT cells overexpressing BR-C. D, inhibition of PKA in BR-C-overexpressing HEK293FT cells. E, the dissected fat body of the wandering silkworm was treated with cAMP or forskolin ex vivo for 6 h and subsequently homogenized for a Western-blotting assay. F, inhibition of the PKA pathway ex vivo. All Western-blotting signals were analyzed, and densitometric analysis of the protein bands from the Western blots was performed using ImageJ software. Values are represented as the mean ± S.E. (error bars); *, p < 0.05; **, p < 0.01 versus controls.