Figure 1.
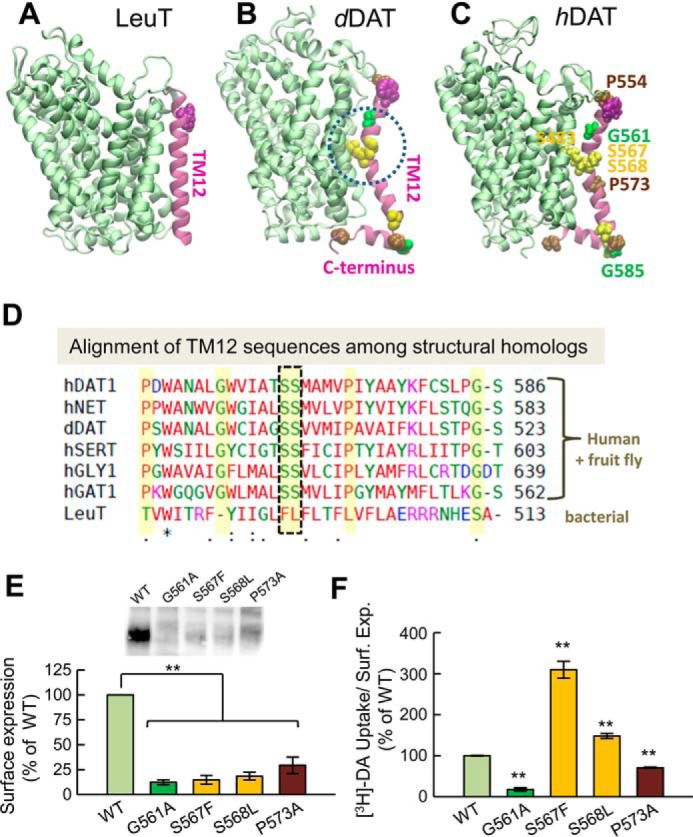
The kink in the TM12 segment of DAT and its functional significance. Structural and sequence differences between LeuT from Aquifex aeolicus (A), dDAT from D. melanogaster (B), and hDAT (human) (C). There is a kink (dashed circle in B) in the TM12 segment of eukaryotic DATs near two serines conserved among the eukaryotic transporters. DAT has an additional C-terminal helix, with a glycine (G585 in hDAT) conserved among human paralogs. Notably, Ser-568 forms hydrogen bonding with Ser-483 in the TM10 segment. D, sequence alignment of TM12 in different members of transporters that share the LeuT fold. The sequences belong to human paralogs hDAT, hNET, hSERT, hGLY1, and hGAT1 and the fruit fly DAT (dDAT) shown in B. The last sequence is that of bacterial LeuT (shown in A). Selected residues conserved among all human paralogs are highlighted in yellow (also labeled in panel C). The kink-forming serines on TM12 are highlighted and enclosed in a dashed box. E and F, effect of mutations at TM12 kink-forming residues on DAT expression and DA uptake. E, surface expression of WT, G561A, S567F, S568L, and P573A hDAT. Transfected HEK293 cells were incubated with sulfo-NHS-SS-biotin and isolated with Neutravidin-agarose beads after cell lysis. For detection of DAT protein an anti-DAT (rat, Millipore MAB369) antibody was used. Densitometry analysis was performed with Image Lab (Bio-Rad) and SigmaPlot 12.5 (Systat Software). Bars represent the percentage of the mean value of the WT group (mean ± S.E.). F, transport activity. Uptake of [3H]DA was performed in HEK293 cells. DA accumulation was normalized with respect to the percentage of cell-surface expression (Surf. Exp.) for each mutant and expressed as the percentage of WT. The statistical analysis was performed with a two-tailed Student's t test versus WT group with an accepted significance level of p < 0.05 (**).
