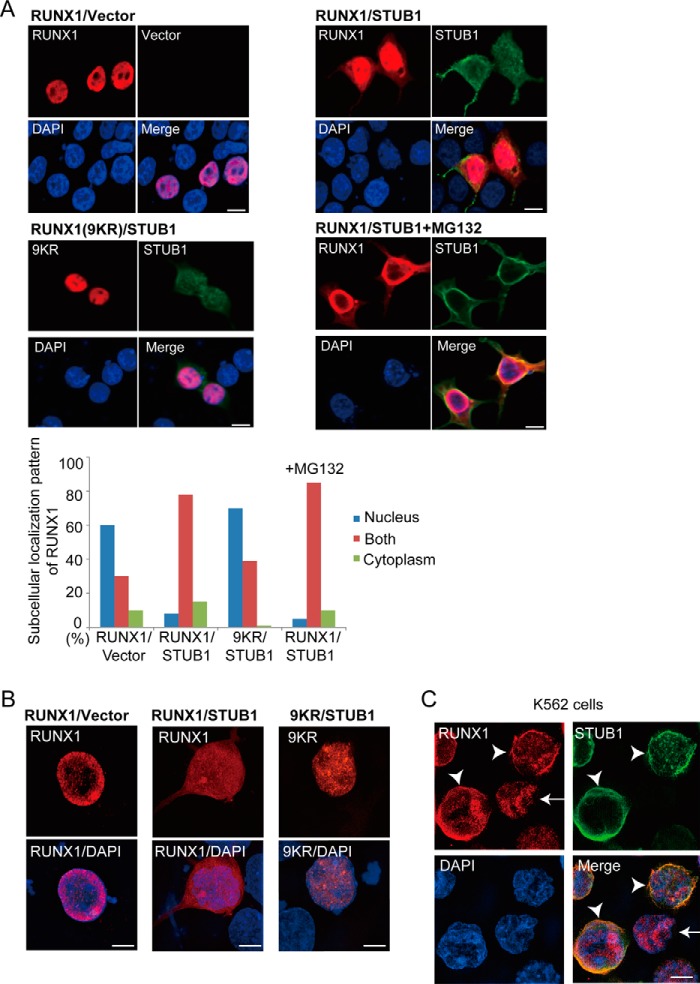
Figure 4.
STUB1 promoted nuclear export of RUNX1. A, 293T cells were cotransfected with RUNX1 (wild-type or the 9KR mutant) together with vector or FLAG-tagged STUB1, and stained with anti-RUNX1 (rabbit) and anti-FLAG (mouse) antibody followed by anti-rabbit Alexa 568 (red) and anti-mouse Alexa 488 (green) staining. Nuclei were visualized with DAPI (blue). Bar, 10 μm. Confocal laser scanning microscopy (Nikon A1) was used to observe localization of RUNX1 and STUB1. Note that RUNX1 displayed a more diffuse localization pattern in both nucleus and cytoplasm in many STUB1-transduced cells. This effect was not observed for the 9KR mutant. Addition of MG132 (20 μm) did not change the RUNX1 localization. Subcellular localization of RUNX1 was quantified by counting the number of cells exhibiting nuclear localization (nucleus), diffuse distribution in both nucleus and cytoplasm (both), or cytoplasmic localization (cytoplasm). B, the cells described in A were also analyzed using the super resolution microscopy (Nikon SIM), which clearly showed the diffuse localization of RUNX1 in both nucleus and cytoplasm in STUB1-transduced cells. Bar, 5 μm. C, K562 cells were transduced with FLAG-tagged STUB1 and stained with anti-RUNX1 (rabbit) and anti-FLAG (mouse) antibody followed by anti-rabbit Alexa 568 (red) and anti-mouse Alexa 633 (green) staining. Nuclei were visualized with DAPI (blue). Bar, 5 μm. The super resolution microscopy (Nikon SIM) was used to observe localization of RUNX1 and STUB1. RUNX1 was observed only in the nucleus in cells without STUB1 expression (arrow), whereas RUNX1 was observed in both nucleus and cytoplasm in STUB1-transduced cells (arrowheads).