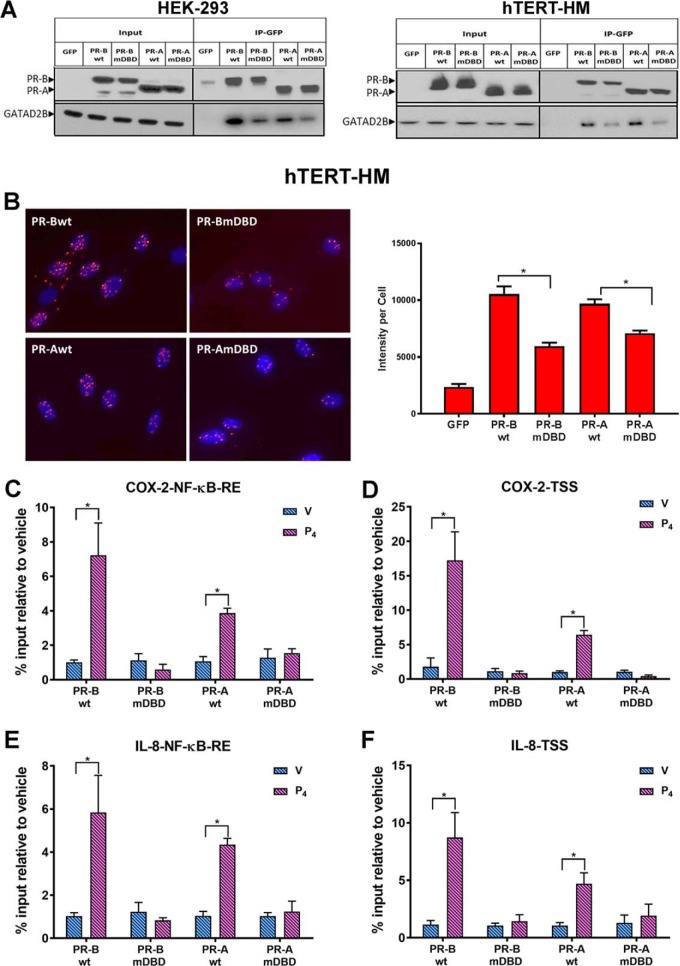
Figure 6.
PR interacts with GATAD2B and recruits GATAD2B to the COX-2 and IL-8 promoters in response to P4 treatment. A, co-immunoprecipitation assays (Co-IP) were performed in stably transfected HEK-293 (left) and in hTERT-HM (right) cells to validate interaction between PR and GATAD2B. Total cell proteins were isolated from cells stably expressing GFP-conjugated PR-BWT, PR-AWT, PR-BmDBD, or PR-AmDBD that had been treated with IL-1β (10 ng/ml) + P4 (1 nm) for 12 h and immunoprecipitated with anti-GFP magnetic beads. The eluted samples were collected for immunoblotting analysis of PR and GATAD2B with input as control. B, Duolink proximity ligation assays were used to detect the direct in vivo interaction between PR and GATAD2B. hTERT-HM myometrial cells stably expressing PR-AWT or PR-BWT or the corresponding DBD mutants were treated with IL-1β (10 ng/ml) + P4 (100 nm) for 40 min. Intensity of fluorescent signal per cell was calculated by normalizing red florescent PLA signals to cell number, which was quantified by DAPI signals (blue) in that image. Data are expressed as mean ± S.E. for each treatment group (n = 6 fields/sample). C–F, ChIP assays were used to analyze the recruitment of GATD2B to an NF-κB response element or TSS in the COX-2 (C and D) and IL-8 gene (E and F) promoters. The myometrial cells were treated with DMSO (V), or P4 (100 nm) for 40 min. qPCR was used to quantify the recruitment of each factor to the promoter using specific primers (Table 1). -Fold recruitment is depicted as percentage input relative to the corresponding vehicle-treated sample. Data are the mean ± S.E. (error bars) of three replicate determinations for each treatment group. *, significant (p < 0.05) difference between samples.