Figure 7.
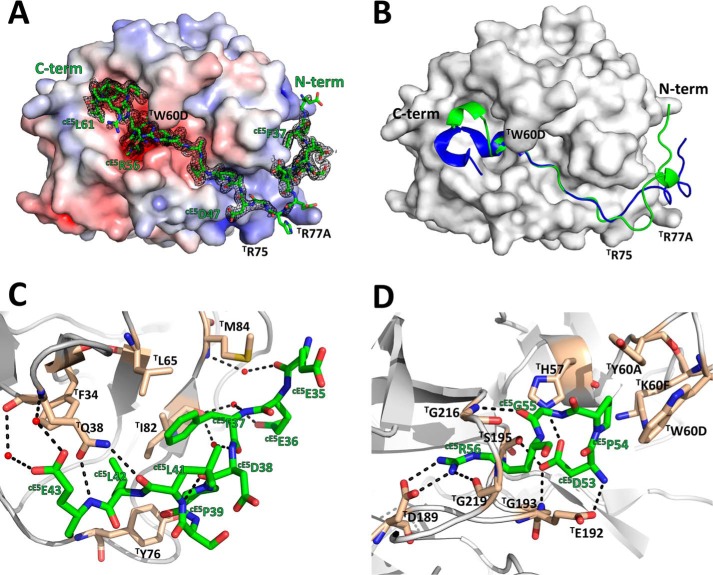
The A. gambiae cE5 anticoagulant blocks both the active site and the exosite I regions of human α-thrombin. A, the acidic cE5(Glu-35–Asp-47) segment of cE5 (stick model with nitrogen atoms in blue, oxygen in red, and carbon in green) binds to the exosite I of thrombin (solid surface representation with positive surface electrostatic potential in blue and negative surface electrostatic potential in red), whereas the downstream segment, up to cE5(Arg-62), threads along the active site cleft of the proteinase, blocking the active site and the non-primed specificity subsites. The 2Fo − Fc electron density map (1.0-σ contour) for cE5 is represented as a black mesh. B, comparison between the A. gambiae cE5 P5 fragment (green) and A. albimanus anophelin (PDB entry 4E05; Ref. 10; blue) shown in schematic representation. Thrombin, shown as a solid white surface, is in the same orientation as in panel A. C, close-up view of the interactions established between the N-terminal segment cE5(Glu-35–Glu-43) of cE5 (colored as in panel A) and the exosite I of thrombin (gray schematic with selected residues as sticks color-coded as cE5, except for carbon atoms, colored tan). Water molecules and hydrogen bonds are represented as red spheres and dotted black lines, respectively. D, close-up view of the interaction between the strictly conserved cE5(Arg-53–Arg-56) tetrapeptide and the active-site region of thrombin (colors as in panel C). The figure was prepared with PyMOL (Schrödinger).