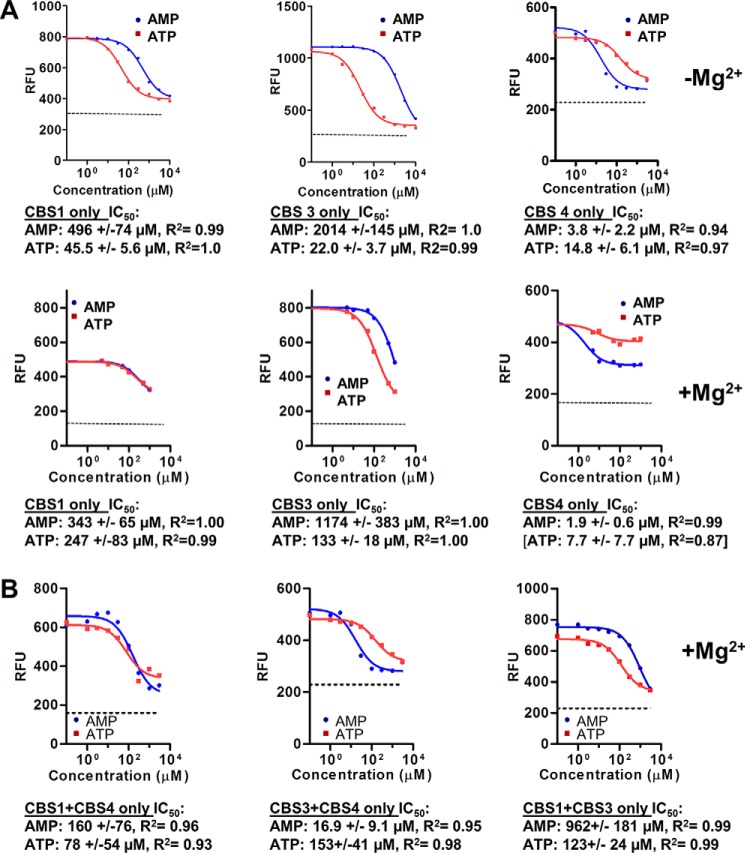
Figure 5.
Nucleotide binding to individual CBSs and pairs of CBSs. Competition of 0.5 μm deac-ADP bound to 4 μm α1β2γ1 AMPK by unlabeled AMP and ATP. A, AMPK mutants with only a single functional adenine nucleotide-binding site (see Fig. 1). Note that in these experiments singly and doubly mutated CBS4 (I312D and I312D/S314A) behaved identically. The ability of Mg2+-AMP to compete deac-ADP from 4 μm AMPK with an IC50 of 1.9 μm indicates that AMP still binds CBS4 with submicromolar affinity, but no longer non-exchangeably. B, AMPK mutants with two functional adenine nucleotide-binding sites. Samples were incubated for 30 min, excited at 430 nm, and emission recorded at 470 nm. The dashed line indicates the fluorescence signal of unbound (i.e. completely competed) deac-ADP.