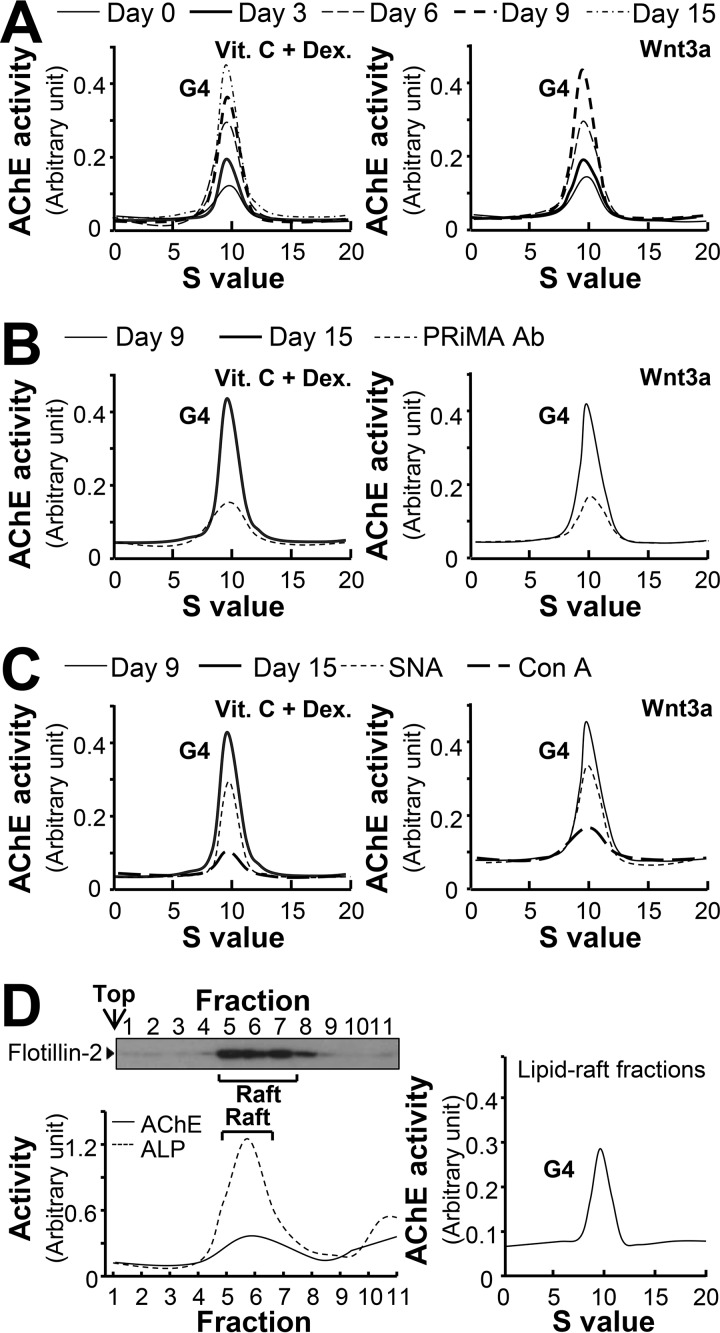
Figure 3.
The G4 form of AChE remains unchanged during osteoblastic differentiation. A, primary cultured osteoblasts were treated with dexamethasone plus vitamin C (Dex. 20 nm, and Vit. C 250 μm) or Wnt3a (200 ng/ml) for a 3-day interval, cell lysates were collected on different days as indicated and subjected to sucrose density gradient analysis. B, equal amounts of proteins from differentiated cell lysates (day 9 and 15) were immune precipitated by anti-PRiMA antibody. C, equal amounts of proteins from differentiated cell lysates, the same control samples (day 9 and day 15) as in B, were incubated with or without Con A or SNA overnight, unbound and lectin-precipitated parts were separated by centrifugation. Supernatant from B and C was subjected to sucrose density gradient analysis. D, left panel: AChE activity from osteoblast membranes in detergent-resistant (Raft; fractions 5–8) and detergent-soluble (non-raft) fractions was determined after flotation in discontinuous sucrose gradients with 0.5% cold Triton X-100. Aliquots of each even fraction were analyzed by 8% SDS-PAGE, and the expression of flotillin-2 (∼55 kDa) was shown in Western blots as control (upper panel). Enzymatic activities of AChE and ALP are expressed in arbitrary units (lower panel). Right panel, sedimentation profile of AChE solubilized from the raft-enriched fractions of osteoblast was determined. Representative gradient profiles from four independent experiments are shown.