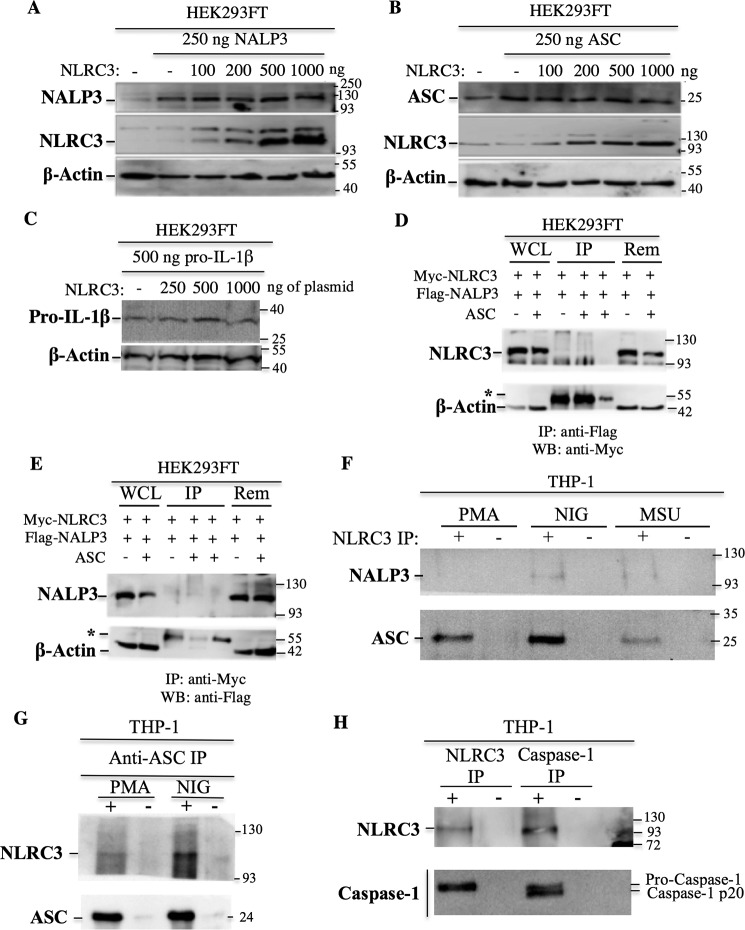
Figure 3.
NLRC3 interacts endogenously with pro-caspase-1 via its CARD and with ASC but not with NALP3. A–C, NLRC3 does not regulate the protein levels of the inflammasome components. Equal amounts of the NALP3 (A), ASC (B), and pro-IL-1β (C) proteins were transfected into HEK293FT cells in the presence of increasing concentrations of NLRC3. Protein levels were analyzed by Western blotting. D and E, 5 × 106 HEK293FT cells were transfected with 6 μg of ASC, Myc-NLRC3, and FLAG-NALP3 plasmids. An irrelevant anti-HA antibody was used as a negative IP control. WCL, whole-cell lysates; Rem, remaining flow-through fraction after IP; asterisks, IgG heavy chains (55 kDa); WB, Western blot. Results from anti-FLAG (D) and anti-Myc (E) are shown. F, interaction of endogenous NLRC3 with endogenous NALP3 and endogenous ASC. IP was performed with anti-NLRC3 antibody in PMA-differentiated THP-1 cells and nigericin (NIG)- and MSU-treated THP-1 cells. G, interaction of endogenous NLRC3 with endogenous ASC. IP was performed with anti-ASC antibody in control and nigericin-treated THP-1 cells. H, interaction of endogenous NLRC3 with endogenous caspase-1 in THP-1 cells. +, IP with anti-NLRC3 or anti–caspase-1 antibody; −, IP with anti-rabbit IgG antibody.