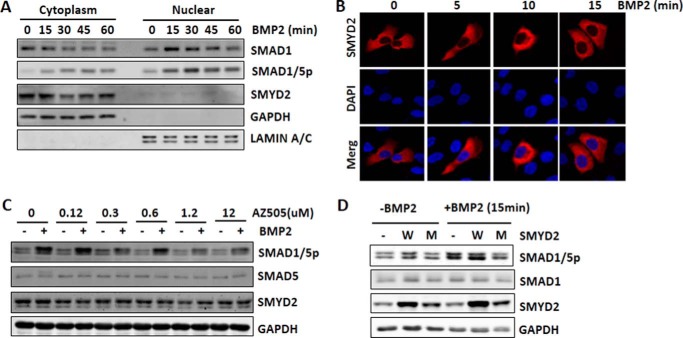
Figure 5.
SMYD2 regulates BMP signaling transduction in cytoplasm in a KMT activity-dependent manner. A, nucleocytoplasmic fractionation showing that SMYD2 remained in cytoplasm regardless of BMP treatment. HaCaT cells were serum-starved overnight, treated with 25 ng/ml BMP2 for various times as indicated, and then subjected to nucleocytoplasmic fractionation and Western blotting with the antibodies against SMAD1, phosphorylated SMAD1/5, and SMYD2. GAPDH and lamin A/C served as control for the cytoplasmic and nuclear fraction, respectively. B, immunofluorescent staining shows that SMYD2 remained in cytoplasm upon BMP treatment. HaCaT cells were transfected with FLAG-SMYD2 for 24 h followed by serum starvation overnight. The cells were then treated with 25 ng/ml BMP2 for various times as indicated and subjected to immunofluorescent staining using anti-FLAG antibody. Nuclei were revealed by DAPI staining. C, SMYD2 selective inhibitor AZ505 diminished BMP2-induced SMAD1/5 phosphorylation. HaCaT cells were serum-starved for 4 h and then treated with increasing concentrations of SMYD2 inhibitor AZ505 for 6 h. The cells were then treated with (+) or without (−) 25 ng/ml BMP2 for 1 h before harvesting for Western blot analysis using antibodies as indicated. Note that BMP2-induced SMAD1/5 phosphorylation was inhibited by addition of 1.2 and 12 μm AZ505. D, enzymatic activity-deficient SMYD2 acted as dominant-negative inhibitor of the BMP signaling. HaCaT cells were transfected with ppY-CAGIP plasmids encoding the wild-type (W) or SMYD2 Y240A mutant (M) at conditions of high transfection efficiency. Two days later, the cells were treated without or with 25 ng/ml BMP2 for 15 min before harvesting for Western blot analysis using antibodies as indicated.