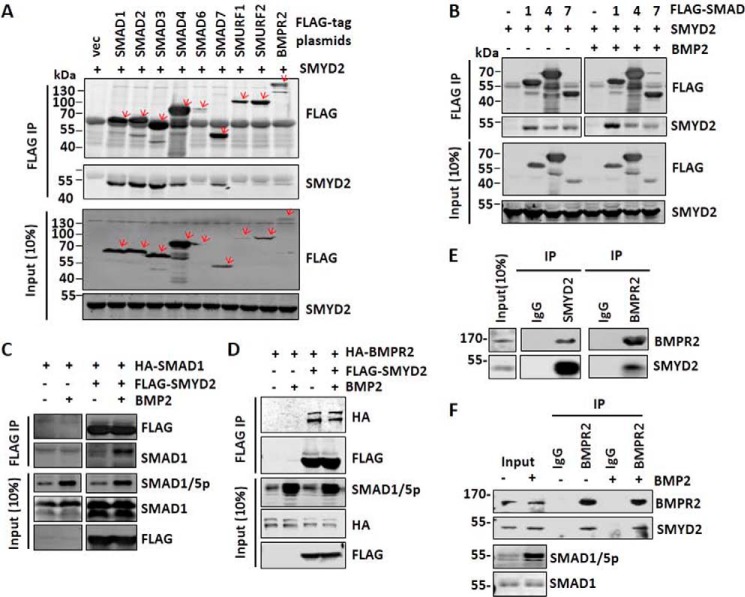
Figure 6.
SMYD2 interacts with multiple components of the TGFβ/BMP-signaling pathways. A, co-immunoprecipitation assay showing interaction between SMYD2 and various components of the TGFβ/BMP-signaling pathways. 293T cells were co-transfected with SMYD2 and FLAG-tagged SMADs, SMURFs, and BMPR2. Two days later, the cells were harvested for immunoprecipitations (IP) using anti-FLAG antibody, followed by Western blot analysis using anti-FLAG and SMYD2 antibodies. B, co-immunoprecipitation assay showing increased interaction between SMYD2 with SMAD1 upon BMP2 treatment. 293T cells were co-transfected with SMYD2 and FLAG-SMAD1, FLAG-SMAD4, or FLAG-SMAD7 for 24 h. The cells were serum-starved overnight and treated with (+) or without (−) 25 ng/ml BMP2 for 1 h, followed by co-immunoprecipitation assay using anti-FLAG antibody. The co-immunoprecipitated SMYD2 was detected by Western blot analysis. Note that BMP treatment only enhanced the SMYD2-SMAD1 interaction. C, reciprocal co-immunoprecipitation assay confirmed a BMP-induced interaction between SMYD2 and SMAD1. 293T cells were co-transfected with FLAG-SMYD2 and HA-SMAD1 for 24 h, serum-starved overnight, and treated with or without 25 ng/ml BMP2 for 1 h, followed by co-immunoprecipitation assay using antibodies as indicated. D, BMP2 treatment did not affect the interaction between SMYD2 with BMPR2. 293T cells were co-transfected with FLAG-SMYD2 and HA-BMPR2 for 24 h, serum-starved overnight, and treated with or without 25 ng/ml BMP2 for 1 h, followed by co-immunoprecipitation assay using antibodies as indicated. E, reciprocal co-immunoprecipitation assay showing the interaction between endogenous SMYD2 and BMPR2 in HaCaT cells. F, HaCaT cells were treated without or with 25 ng/ml BMP2 for 1 h before harvesting for co-immunoprecipitation assay as above.