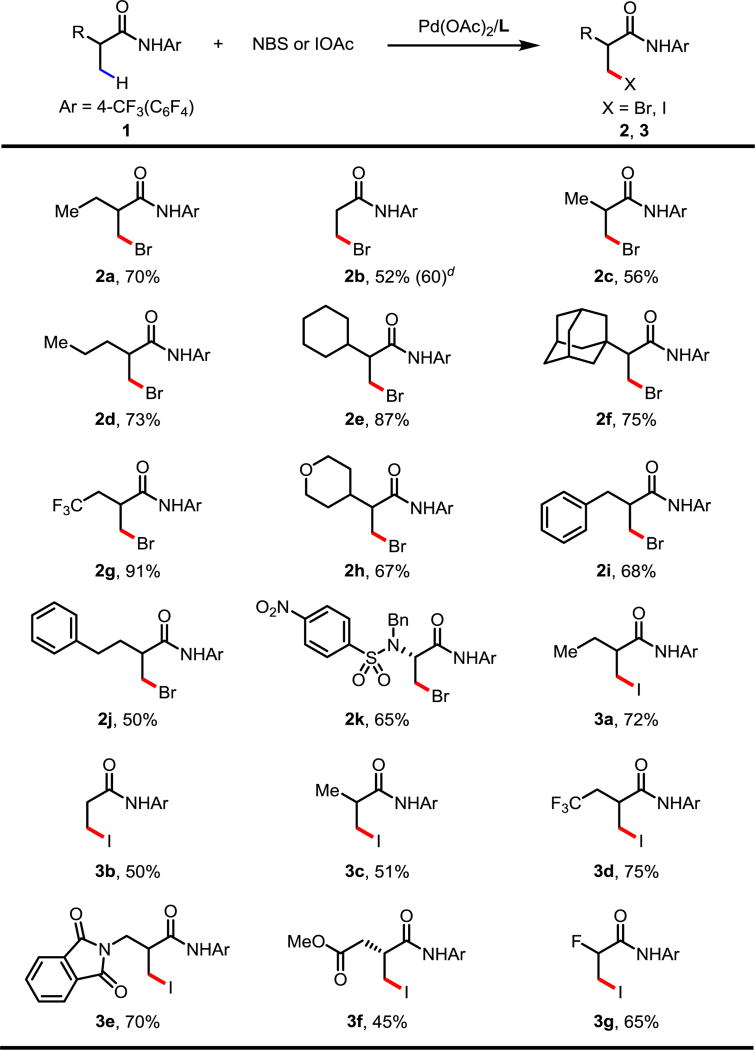
Table 3.
Bromination conditions: 0.1 mmol of 1a, 1.75 equiv of NBS, 10 mol% of Pd(OAc)2, 10 mol% of ligand L6, 1.5 equiv of PhI(OAc)2, 5.0 equiv of AcOH, 1.0 mL of DCE, 70 °C, under air, 20 h.
Iodination conditions: 0.1 mmol of 1a, 1.5 equiv of I2, 1.5 equiv of PhI(OAc)2, 10 mol% of Pd(OAc)2, 10 mol% of ligand L17, 1.0 equiv of AcOH, 0.8 mL of 1,4-dioxane, 0.2 mL of DCE, 80 °C, under air, 20 h.
Isolated yields.
2.0 mmol scale.