Abstract
Malnutrition is a known complication of chronic GVHD (cGVHD), but has not been well described in the context of organ-specific manifestations and the recent National Institutes of Health (NIH) criteria. Here, 210 cGVHD patients were analyzed, in a cross-sectional study design, for demographics, transplant-related history, clinical assessments, symptoms, function, quality-of-life, laboratory values and survival in order to determine their associations with nutritional status. Most patients had long-standing, moderate or severe cGVHD and had failed many lines of therapy. Twenty-nine percent (60/210) of subjects were malnourished, using the subjective Patient-Generated Subjective Global Assessment (PG-SGA) questionnaire and evaluation. No demographic or transplant characteristics were associated with malnutrition; cGVHD of the lungs, gastrointestinal (GI) tract and mouth, NIH global score, cGVHD symptoms, worse functioning, low albumin, poorer survival and low BMI were associated with malnutrition. A predictive model was developed from all variables of significance: cGVHD of the lungs, GI tract, mouth and BMI accurately predicted 84.2% of malnourished patients as well as 87.2% of well-nourished patients. The PG-SGA questionnaire may be a useful tool in diagnosing nutritional deficits in cGVHD patients undergoing one-time evaluations. Longitudinal prospective studies should assess the utility of nutritional support interventions in cGVHD.
Introduction
Chronic GVHD (cGVHD) is a unique late effect of cancer therapy and a major complication after allogeneic hematopoietic SCT (HSCT), resulting in immunodeficiency, impaired organ function and decreased survival.1,2 It commonly affects the skin, mouth, eyes, muscle, fascia, joints, gastrointestinal (GI) tract, liver and lungs, often resembling autoimmune disease, such as scleroderma, Sjögren’s syndrome, primary biliary cirrhosis, bronchiolitis obliterans or immune cytopenias.3
Eating problems and malnutrition are complications associated with cGVHD and contribute to functional and health status impairments in transplant survivors.4 However, the comprehensive analysis of nutritional status and its relationship to cGVHD and other clinical outcomes has not been done. Cancer-related cachexia is highly associated with reduced survival,5 and non-relapse mortality for underweight patients at HSCT seems greater than for healthy-weight patients.6 Patients with cGVHD often have low BMI or low lean BMI.4,7 The use of BMI as a measure of malnutrition, however, is problematic, owing to the wide variation of body composition and pre-existing nutritional status among individuals.8 The Patient-Generated Subjective Global Assessment (PG-SGA), based on patient-reported questionnaire, medical history and physical examination, is the only malnutrition-screening tool recommended by the American Society of Parentral and Enteral Nutrition (ASPEN) in cancer,9 but has not yet been used in the context of cGVHD.
The current study describes malnutrition in a large cohort of adult cGVHD patients, most with long-standing moderate to severe cGVHD and with multiple failed lines of prior therapy. A comprehensive analysis was conducted to address how clinical and cGVHD-related factors impact nutritional status. The study emphasizes the importance of nutritional evaluation in cGVHD, showing that malnutrition is strongly associated with serious cGVHD manifestations and functional and quality-of-life (QOL) impairments.
Methods
Patients
Patients in this study were enrolled in an ongoing cGVHD natural history study at the National Institutes of Health (NIH) (clinicaltrials.gov #NCT00331968). They were referred to the NIH for evaluation and enrolled to into the study if they had cGVHD according to the NIH Consensus Group Criteria definition.3 Subjects underwent a 4-day, one-time visit evaluation by a multi-disciplinary team of clinical experts in dermatology, ophthalmology, dentistry, rehabilitation medicine, gynecology, pain and palliative care and HSCT management. Clinical assessments, patient-reported forms and laboratory data were recorded using pre-defined data collection instruments. Survival data were obtained by follow-up calls to the patients or their referring physician offices. All patients signed NCI Institutional Review Board-approved informed consents.
Nutritional evaluation
BMI was calculated for all patients, based on their height and weight at the time of evaluation.10 The PG-SGA score and global assessment category was recorded for every subject by a certified nutritionist (Supplementary Figure 1). The PG-SGA provides a total score based on information from the patients and the nutritionist evaluator, detailing weight history, dietary intake, nutritional impact of symptoms, functioning, disease state, physical evaluation, metabolic and physical demands, and is used to provide nutritional triage recommendations. The nutritionist assigns a global assessment category of nutritional status (well nourished, moderately or suspected malnourished or severely malnourished).9 For this study, nutritional global assessment was dichotomized to either well-nourished vs malnourished groups (including moderately and severely malnourished groups). Recommendations for nutritional intervention and treatment needs based on PG-SGA results were either followed through the NIH nutrition department or provided to the patient and referring physician.
Measures and study instruments
Demographic information, HSCT-related information and cGVHD-related data were documented for all subjects. Clinician assessment of the organ systems affected by cGVHD were scored using the NIH Organ Scoring of cGVHD form, including skin, eyes, mouth, GI tract, liver, lungs, joints and fascia and the female genital tract;3 the NIH Clinical Activity Assessments for GI, lung and oral cGVHD were also analyzed.11 Patient-reported symptoms were elicited using the cGVHD-specific Lee Symptoms Scale (0–100), which captures the severity of bother by symptoms important in cGVHD, including eating/digestion and eyes/mouth subscales of symptom intensity.12 An NIH cGVHD activity assessment patient-reported form also gathered information on symptomatic mouth dryness and mouth pain, each based on a 0–10 scale.11 Functional and QOL evaluations were performed using the Human Activity Profile instrument,13 the Medical Outcomes SF-36 instrument14 and the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) instrument,15 all validated for cGVHD in HSCT studies.16, 17, 18 Routine laboratory blood analysis was done by the Department of Laboratory Medicine, Clinical Center, NIH.
Statistical analysis
The primary study objective was to identify factors that are associated with malnutrition in a cross-sectional cGVHD subject group. Univariate analyses were performed to screen for factors to evaluate in a multivariable model. Dichotomous parameters were compared between patients with and without malnutrition using Fisher’s exact test. Categorical parameters were compared using Mehta’s modification of Fisher’s exact test.19 Ordered categorical parameters were compared using a Cochran–Armitage test for trend.20 Continuous parameters were compared between groups using an exact Wilcoxon rank-sum test. Those factors that are associated with malnutrition status with a univariate P-value <0.05 were evaluated in univariate logistic regression models and included in a multivariable logistic regression analysis model if P<0.01 in the univariate model. The final multivariable model was determined using backward selection as well as other considerations such as amount of missing data. All P-values from univariate analyses are two-tailed and presented without adjustment for multiple comparison. In the context of an exploratory analysis, and in view of the large number of tests performed, only P-values <0.001 are considered potentially statistically significant with respect to the individual univariate results.
A survival analysis of nutritional status was initially performed with Kaplan–Meier curves, starting at the date the patients enrolled in the natural history protocol. The difference between pairs of Kaplan–Meier curves was determined by a two-tailed log-rank test. Then, a multivariable Cox proportional hazards model was developed, based on six other parameters that have been previously identified as being of potential importance to survival in our patient group (NIH lung score, lung function score, forced expiratory volume in the first second, body surface area for skin erythema, absolute lymphocyte count and Karnofsky score21,22) using both stepwise and backward selection. The resulting models had nutritional status added as a variable to test for its independent association with survival after adjusting for the other previously identified factors found to be jointly significant with survival.
Results
Demographic and transplant-related variables
Two-hundred and ten patients, ages 18–70 years (median 49 years), met criteria for diagnosis of cGVHD and had a nutritional evaluation (Table 1).3 Initial screening was done on 255 patients; 38 patients were excluded from this analysis because they did not receive a nutrition evaluation (including 21 pediatric patients) and 7 were ineligible for study participation, as their evaluation ruled out cGVHD. Demographic and transplant-related variables were analyzed, and no statistically significant association with malnutrition within this group of variables was found, except for number of lines of prior systemic therapy for cGVHD (P=0.013).
Table 1.
Patient and cGVHD characteristics
| Patient characteristics | N (%) |
|---|---|
| Total number of patients | 210 |
| Age (years) | 49 (18–70) |
| Gender | |
| Male | 115 (55%) |
| Female | 95 (45%) |
| Disease | |
| ALL/AML/MDS | 85 (40%) |
| Lymphoma/CLL/MM | 79 (38%) |
| CML | 34 (16%) |
| Sarcoma | 2 (1%) |
| Aplastic anemia/PNH | 7 (3%) |
| Other non-malignant | 3 (2%) |
| Conditioning regimen | |
| Myeloblative | 109 (52%) |
| TBI | 76 (36%) |
| Unknown | 25 (12%) |
| Donor relationship | |
| Unrelated | 73 (35%) |
| Related | 137 (65%) |
| Cell source | |
| BM | 38 (18%) |
| Peripheral blood | 169 (81%) |
| Cord blood | 3 (1%) |
| HLA match | |
| Yes | 173 (82%) |
| No | 31 (15%) |
| Unknown | 6 (3%) |
| cGVHD onset type | |
| Progressive | 83 (40%) |
| Quiescent | 50 (24%) |
| De novo | 74 (35%) |
| Unknown | 3 (1%) |
| Activity by therapeutic intenta | |
| Active | 99 (47%) |
| Not active | 71 (34%) |
| Not on immunosuppression | 29 (14%) |
| Not applicable | 11 (5%) |
| Intensity of immunosuppressionb | |
| None/mild | 55 (26%) |
| Moderate | 79 (38%) |
| High | 76 (36%) |
| Lines of prior systemic therapy for cGVHD treatment | 3(0–9) |
| Organ involvementc | |
| Skin | 165 (79%) |
| Mouth | 143 (68%) |
| Eyes | 170 (81%) |
| Gl tract | 92 (44%) |
| Liver | 109 (52%) |
| Lungs | 162 (78%) |
| Joint/fascia | 130 (62%) |
| Female genital tract | 47 (49%) |
| NIH global organ scored | |
| Mild | 4 (2%) |
| Moderate | 69 (33%) |
| Severe | 137 (65%) |
| NIH average scoree | 1.00 (0.14–2.14) |
| Median number of years from transplant to study enrollment | 2.78 (0.34–21.11) |
| Median number of years from cGVHD diagnosis to study enrollment | 1.88 (0–18.27) |
Nutritional status in cGVHD
BMI, the PG-SGA and its component scores are shown in Table 2. When malnourishment was defined by a nutrition global assessment of moderately or severely malnourished (stages B plus C categories of the PG-SGA global assessment), then 71.4% of our cohort were well nourished and 29.5% were malnourished (23.3% moderately and 5.2% severely) (Figure 1a). This approaches the 27.2% incidence of malnourishment as defined by BMI<21; however, if BMI is compared with nutrition global assessment (Figure 1b), it is apparent that these two measures are non-synonymous. There is a mismatch between nutritional evaluation and BMI that can be seen when analyzing well-nourished patients, where 14 of 141 (10%) had a BMI ≤ 21, or when analyzing malnourished patients, 42 of 59 (71%) of whom had a BMI ≤ 21, showing overlap among groups.
Table 2.
PG-SGA score in cGVHD
| Evaluation | Mean±s.e.m. | |
|---|---|---|
| BMI | 24.56 ± 0.35 | |
| Patient-Generated Subjective Global Assessment Score (PG-SGA Score)a | Total PG-SGA score | 7.02 ±0.36 |
| Weight summary | 0.42 ±0.07 | |
| Food intake | 0.37 ±0.06 | |
| Symptoms | 2.56 ±0.22 | |
| Activities and function | 1.15 ±0.07 | |
| Disease requirements | 1.02 ±0.02 | |
| Metabolic demand | 0.91 ±0.08 | |
| Physical | 0.60 ±0.07 | |
| Nutrition Global Assessment (PG-SGA)b | Well-nourished | N= 150 (71.4%) |
| Moderate or suspected malnutrition | N=49 (23.3%) | |
| Severely malnourished | N=11 (5.2%) |
Abbreviation: cGVHD = chronic GVHD.
A total PG-SGA score of 2 or above indicates the need for nutritional intervention, including patient and family education, symptom management including pharmacologic intervention, and appropriate nutrient intervention. For the component scores (weight summary, food intake, symptoms, activities and function, disease requirements, metabolic demand and physical), any deviation from zero indicates a deficit from optimal nutrition.
PG-SGA Global Assessment categories are based on clinician evaluation of patient’s weight, nutritional intake, nutrition impact of symptoms, functioning and physical exam. The categories are well-nourished, moderately malnourished or suspected malnutrition, or severely malnourished: for this study, groups were made of well nourished vs malnourished (moderately malnourished and severely malnourished).
Figure 1.
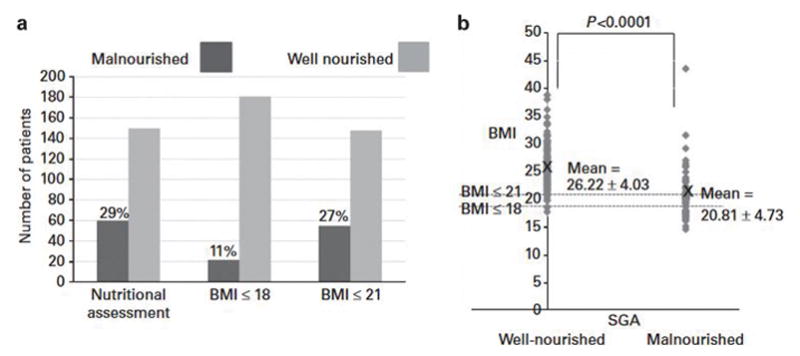
Malnutrition in cGVHD. The prevalence of malnutrition in cGVHD, as defined by PG-SGA questionnaire and BMI (a). Mean BMI is lower in the malnourished group than in the well-nourished group as defined by PG-SGA; however, substantial overlap of BMIs between groups suggests that the two measures are non-synonymous (b).
Assessment of organ systems
Of the organ systems affected by cGVHD, lung, GI tract and mouth cGVHD were found to be significantly associated with malnutrition (P<0.0001, Table 3). The NIH average organ score, which synthesizes clinical assessments, and the Health Care Provider Global Rating (mild-moderate-severe) were also significant (P<0.0001). Skin, eyes, liver and female genital tract NIH organ scores were not associated with malnutrition. The cGVHD Total Lee Symptoms Scale was associated with malnutrition (P<0.0001, Table 3).
Table 3.
Variables significantly associated with malnutrition in univariate analysis
| Instrument | Number of subjects (% malnourished) | P-value | |
|---|---|---|---|
| Categorical variables | |||
| Global NIH Score | 1 = 4 (0%), 2 = 68 (15%), 3 = 137 (37%) | 0.0006 | |
| Joints and fascia NIH Score | 0 = 81 (22%), 1 = 47 (28%), 2 = 60 (30%), 3 = 21 (52%) | 0.02 | |
| Lungs NIH Score | 0 = 48 (12%), 1 = 87 (25%), 2 = 51 (33%), 3 = 21 (71%) | < 0.0001 | |
| Gl tract NIH Score | 0 = 117 (15%), 1 = 69 (33%), 2 = 16 (88%), 3 = 7 (86%) | < 0.0001 | |
| Mouth NIH Score | 0 = 65 (17%), 1 = 115 (28%), 2 = 23 (52%), 3 = 6 (83%) | < 0.0001 | |
| Physician global rating of cGVHD activity | 1 = 38 (11%), 2 = 67 (25%), 3 = 67 (45%) | 0.0002 | |
| Instrument | Well-nourished (mean ± s.e.m.) | Malnourished (mean ± s.e.m.) | P-value |
|
| |||
| Continuous variables | |||
| Lines of prior systemic therapy for cGVHD | 3.43 ± 0.16 | 4.23 ± 0.26 | 0.01 |
| NIH Average Score | 0.96 ± 0.03 | 1.28 ± 0.05 | < 0.0001 |
| Lee total Scorea | 32.78 ± 1.33 | 42.74 ± 2.09 | < 0.0001 |
| SF-36 physical health summaryb | 38.04 ± 0.86 | 31.06 ± 1.56 | < 0.0001 |
| FACT-BMT physical well-beingc | 19.30 ± 0.53 | 17.22 ± 0.82 | 0.02 |
| FACT-BMT functional well-beingc | 17.35 ± 0.54 | 13.79 ± 0.86 | 0.0006 |
| HAP, Maximum Activity Score (MAS)d | 73.93 ± 1.09 | 63.24 ± 2.29 | 0.0001 |
| HAP, Adjusted Activity Score (AAS)d | 63.08 ± 1.40 | 49.69 ± 2.86 | < 0.0001 |
| Albumin | 3.66 ± 0.04 | 3.45 ± 0.06 | 0.004 |
| BMI (kg/m2) | 26.17 ± 034 | 20.84 ± 0.62 | < 0.0001 |
Abbreviations: cGVHD = chronic GVHD; FACT-BMT = Functional Assessment of Cancer Therapy-Bone Marrow Transplant; HAP = Human Activity Profile; NIH = National Institutes of Health; SF-36 = Short-Form 36 Health Survey.
The Lee cGVHD Symptom Scale is a patient-reported questionnaire with seven subscales (skin, eyes and mouth, breathing, eating and digestion, muscles and joints, energy, mental and emotional) designed to capture cGVHD-specific symptom burden.
The FACT-BMT, is a patient-reported questionnaire concerning BM-related quality-of-life issues. It has four domains (physical well-being, social/family well-being, emotional well-being and functional well-being).
The SF-36 Health Survey is a patient-reported questionnaire concerning health quality-of-life issues. It has eight sections (physical functioning, bodily pain, general health perceptions, physical role functioning, vitality, emotional role functioning, social role functioning and mental health). The first four sections are combined as a Physical Health Summary, and the second four sections are combined as a Mental Health Summary.
The HAP is a patient-reported questionnaire of energy expenditure or physical fitness.
All aspects of GI tract pathology, both for clinical assessment and for symptom reporting, were associated with malnutrition (NIH Clinical Activity Assessment: GI upper, P<0.0001, GI esophageal, P=0.0025, GI lower, P=0.0081, Lee Eating and Digestion Subtotal and individual questions) (Table 4). Clinical assessment of lung function (Lung NIH Clinical Activity Assessment: P<0.0001), as well as Lee shortness of breath with exercise (P<0.0001) was found to be significantly associated. Mouth cGVHD clinical assessments (NIH Oral Mucosal Score: Clinical Activity Assessment: P=0.03) and certain mouth pain questions (Lee need to avoid food due to mouth pain: P=0.009, mouth pain=0.31) tended to be significant, but none met the criteria of P<0.001 (Table 4).
Table 4.
Gastrointestinal, lung, and mouth associations with malnutrition in univariate analysis
| Instrument | Number of subjects (% malnourished) or mean±s.e.m. (malnourished vs wel nourished) | P-value |
|---|---|---|
| Gastrointestinal cGVHD | ||
| Upper GI: NIH Clinical Activity Assessmenta | 0 = 30 (22%), 1 = 16 (50%), 2 = 5 (50%), 3 = 3 (100%) | < 0.0001 |
| Esophageal: NIH Clinical Activity Assessmenta | 0 = 31 (25%), 1=11 (37%), 2 = 6 (43%), 3 = 6 (75%) | 0.003 |
| Lower GI: NIH Clinical Activity Assessmenta | 0 = 38 (25%), 1 =21 (57%), 2 = 4 (57%), 3 = 0 (0%) | 0.008 |
| Lee Eating and Digestion Subsection total | 4.00 ± 0.44 vs 1.34 ± 0.17 | < 0.0001 |
| Difficulty swallowing solid foods | 0 = 16(16%), 1 = 14 (36%), 2 = 11 (52%), 3 = 5 (42%), 4 = 8 (61%) | < 0.0001 |
| Difficulty swallowing liquids | 0 = 33 (24%), 1 = 11 (50%), 2 = 6 (54%), 3 = 3 (50%), 4 = 1 (33%) | 0.009 |
| Vomiting | 0 = 39 (25%), 1 = 10 (56%), 2 = 4 (67%), 3 = 0 (0%), 4 = 1 (100%) | < 0.0001 |
| Weight loss | 0 = 25 (19%), 1 = 7 (26%), 2 = 8 (100%), 3 = 4 (50%), 4 = 10 (91%) | < 0.0001 |
| Lung cGVHD | ||
| Lung—NIH Clinical Activity Score | 0 = 23 (20%), 1 = 49 (29%), 2 = 33 (48%), 3 = 6 (75%) | < 0.0001 |
| Lung Function Scoreb | 6.21 ± 0.42 vs 4.80 ± 0.42 | 0.001 |
| Lee Breathing Subsection total | 5.54 ± 0.68 vs 3.59 ± 0.30 | 0.01 |
| Frequent cough | 0 = 27 (29%), 1 = 8 (23%), 2 = 7 (26%), 3 = 7 (39%), 4 = 5 (50) | 0.27 |
| Colored sputum | 0 = 31 (26%), 1 =35 (31%), 2 = 7 (47%), 3 = 1 (14%), 4 = 4 (67%) | 0.08 |
| Shortness of breath with exercise | 0 = 7 (19%), 1 = 8 (19%), 2 = 1 (23%), 3 = 15 (42%), 4 = 14 (56%) | < 0.0001 |
| Shortness of breath at rest | 0 = 38 (29%), 1 = 5 (18%), 2 = 6 (25%), 3 = 4 (67%), 4 = 1 (50%) | 0.20 |
| Need to use oxygen | 0 = 45 (27%), 1 = 1 (50%), 2 = 1 (50%), 3 = 1 (100%), 4 = 6 (67%) | 0.003 |
| Mouth cGVHD | ||
| NIH Oral Mucosal Score: NIH Clinical Activity Assessmentc | 2.60 ± 0.3 vs 1.98 ± 0.17 | 0.03 |
| Lee Eyes and Mouth Subsection total | 9.30 ± 0.76 vs 7.79 ± 0.4 | 0.10 |
| Need to avoid certain foods due to mouth pain | 0 = 17 (22%), 1 = 9 (37%), 2 = 6 (21 %), 3 = 11 (38%), 4 = 10 (55%) | 0.009 |
| Ulcers in mouth | 0 = 30 (27%), 1 = 7 (28%), 2 = 8 (42%), 3 = 6 (24%), 4 = 3 (75%) | 0.34 |
| Mouth dryness (0–10 symptom score) | 2.65 ± 0.29 (vs 3.98 ± 0.44) | 0.004 |
| Mouth pain (0–10 symptom score) | 1.66 ± 0.26(vs 2.22 ± 0.47) | 0.31 |
Abbreviations: cGVHD = chronic GVHD; GI = gastrointestinal tract; NIH = National Institutes of Health.
GI symptoms during the preceding week through interview with clinician according to 0–3 severity scales. Upper GI symptoms include early satiety, anorexia, nausea and vomiting. Esophageal symptoms include dysphagia or odynophagia. Lower GI symptoms include loose or liquid stools or diarrhea.
Lung Function Score is computed using the addition of scores assigned to the forced expiratory volume in the first second (FEV1) and single-breath diffusion lung capacity for carbon monoxide (DLCO) adjusted for Hb (scores: > 80% of predicted = 1, 70–79% = 2, 60–69% = 3, 50–59% = 4, 40–49% = 5, < 40% = 6).
Mucosal changes inside the mouth are scored by a clinician for erythema, lichenoid, ulcers and mucoceles and totaled for a scale of 0–15.
Functional and QOL evaluations
Functional and QOL evaluations were performed and associated with nutritional status. Worsening Human Activity Profile score was malnutrition associated for both the Maximum Activity Score (P=0.0001) and Adjusted Activity Score (P<0.0001).18 SF-36 score components found to be significant at the P<0.005 level included physical functioning, physical role, general health and physical component summary; at the P<0.05 level included vitality, social functioning and mental health. Bodily pain, emotional role and mental component summary were non-significant. For the FACT-BMT, total score (P=0.002), functional well-being (P=0.0006) and physical well-being (P=0.02) were found to be associated with malnutrition, whereas social/family well-being and emotional well-being were not. Table 4 shows variables significantly associated with malnutrition.
Laboratory analysis
Of the 17 laboratory blood values that were studied (erythrocyte sedimentation rate, white blood count, ANC, absolute lymphocyte count, eosinophils, Hb, C-reactive protein, albumin, ferritin, beta-2-microglobulin, total protein, IgG, IgA, C3 complement, C4 complement, total complement and parathyroid hormone), only lower albumin was found to be significantly associated with malnutrition (P=0.0036, Table 4).
Multivariable logistic model for the prediction of malnutrition in cGVHD
Out of over 80 parameters evaluated for their association with malnutrition, ~ 40 were found to be associated with malnutrition (P<0.05), using the above-detailed screening procedures. From these parameters, all but 10 were associated with malnutrition with P<0.01, using a univariate logistic regression model. From these ~30 parameters, 7 were jointly associated with malnutrition using a backward selection model. However, as two parameters had numerous missing data points, the model was rebuilt using only the remaining five parameters, which rendered one non-significant. The final model included four parameters: NIH lung score, NIH GI score, NIH mouth score and BMI (Table 5). This model was converted to a classification rule and applied to the patient cohort, which resulted in the following classification probabilities: of the 133 subjects who were found to be well nourished by nutritional evaluation, 116 subjects (87.2%) would be correctly classified by this rule; of the 57 subjects who were found to be malnourished, 48 (84.2%) would be correctly classified.
Table 5.
Predictive model for cGVHD malnutritiona
| Variable | Well nourished (mean ± s.d.) | Malnourished (mean ± s.d.) | P-value in model |
|---|---|---|---|
| NIH Score lungs | 1.03 ± 0.83 | 1.68 ± 0.96 | 0.0046 |
| NIH Score GI tract | 0.36 ± 0.55 | 1.15 ± 0.95 | 0.0005 |
| NIH Score mouth | 0.72 ± 0.62 | 1.18 ± 0.83 | 0.0116 |
| BMI | 26.17 ± 4.01 | 20.84 ± 4.66 | < 0.0001 |
Abbreviations: cGVHD = chronic GVHD; GI = gastrointestinal tract; NIH = National Institutes of Health. Prediction = −14.096 x NIH score lungs–24.506 x NIH score GI tract–17.597 x NIH score mouth+6.530 x BMI; if prediction ≤100, then prediction of malnourished; if prediction >100, then prediction of well nourished.
Using the parameters from this model, and converting this model to a classification rule results in the following relationship.
Survival analysis
In a univariate analysis, malnourished patients had poorer survival compared with well-nourished patients (P=0.005, Figure 2). The median follow-up for survivors was 38.3 months. After 3 years, the survival probability for malnourished patients was 69% compared with 82% for well-nourished patients; BMI was not found to be associated with survival. After adjusting for subsets of variables that have previously been found to be jointly predictive of survival in this patient group,21,22 through backward and stepwise Cox model construction, malnutrition was no longer independently associated with survival.
Figure 2.
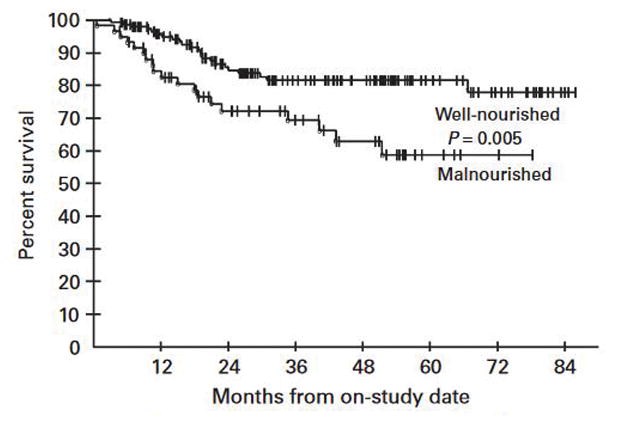
Survival analysis for malnutrition in cGVHD. Malnourished patients have poorer survival compared with well-nourished patients (log-rank, P = 0.005), in univariate analysis. In multivariable analysis, after adjusting for factors associated with survival in this cohort on previous analysis (NIH lung score, Karnofsky score, lymphocyte count, BSA erythema), this association was lost.
Discussion
Eating problems and malnutrition are often significant issues for patients with cGVHD and are associated with nausea, vomiting, diarrhea, mouth pain, dysphagia, dysgeusia, xerostomia, anorexia, early satiety and weight loss.23 We show that malnutrition is a significant consideration in cGVHD diagnosed by NIH criteria, as 29% of our subjects were malnourished per nutritional evaluation. Lenssen et al.24 studied weight loss in HSCT patients 1 year post transplant and reported 33% of patients with extensive cGVHD and 19% with localized cGVHD had weight-loss problems. Jacobsohn et al.4 found that among 93 patients with cGVHD, 43% had a BMI<21.9 kg/m2 and 14% had a BMI<18.5 kg/m2, using this criterion to designate malnourished and severely malnourished, respectively.
The results of the current study are based on a thorough nutritional evaluation using the PG-SGA instrument (Table 2), and eliminate the problems that are inherent with weight-based evaluations, where initial weight is variable, weight loss is often gradual and subtle and the ramifications of low BMI are often a late finding after clinical and QOL issues have already negatively affected the patient.8,23 Disadvantages of the PG-SGA include the specialized training and time needed to correctly administer the tool; however, it is the most widely used and accepted assessment of nutritional status in oncology, and its use could lead to improved therapeutic interventions to improve nutrition.10
This cohort consisted of patients with long-standing cGVHD. Almost half of all patients had active cGVHD, three-quarters had moderate or high levels of current immunosuppressive therapy and most had previously been on prior systemic therapy for cGVHD, showing the resistant nature of their cGVHD. Almost all patients were considered to have moderate or severe cGVHD. Current findings must be placed in this context and will have to be tested in more newly diagnosed and less severe cases to become generalizable.
Symptoms related to cGVHD may have profound impacts on nutritional status, and are one potential mechanism to explain malnutrition in this current patient group. Oral cGVHD findings and symptoms are often cited as being associated, or even the direct cause, of malnutrition, secondary to mouth pain with eating.24, 25, 26, 27, 28 Here is shown that although oral cGVHD is a predictor of malnutrition in cGVHD, most symptoms relating to mouth pain were not, although 37% of the total PG-SGA score was due to the symptoms component. Thus oral cGVHD is associated with malnutrition in cGVHD but it does not appear to be pain mediated. Similar work by Jacobsohn et al.4 showed a lack of association between oral symptoms and weight loss. Further, all symptoms reported as describing GI tract problems (including esophageal pain, dysphagia, diarrhea, problems eating or drinking, vomiting and patient-reported weight loss), are significantly associated with poor nutritional status, as is GI cGVHD assessment.
The other main mechanism driving poor nutritional status in cGVHD may relate to the activity of the cGVHD itself. We find that clinical assessments for oral, lung and GI cGVHD are significantly associated with malnutrition. Other studies have indicated that active cGVHD4,7 and poor performance status as measured by the Karnofsky score7 are linked with nutritional problems. We find that decreased functional status and QOL, as measured by Human Activity Profile, SF-36 and FACT-BMT, are all associated with malnutrition in our cGVHD group. The physical and functional components appear to be associated with malnutrition rather than the emotional, social or mental aspects of these tests. These associations are probably owing to underlying metabolic deficits seen with cGVHD, including increased tissue repair needs, infection defense and possible hypermetabolism, all of which are worsened with standard corticosteroid treatment.28 We did not, however, find a direct association between level of immunosuppression at the time of evaluation and malnutrition.
We also evaluated standard laboratory blood tests as predictors of malnutrition. A recent study by our group (Grkovic et al.21) has shown that lower albumin, higher platelets and higher C-reactive protein were associated with cGVHD activity. In this analysis, only albumin was found to be significantly associated with malnutrition. Although low albumin levels have been used for assessing malnutrition, decreased albumin levels are a marker of disease and inflammation rather than a feature of malnutrition,8,29 and here may act as another indication of high cGVHD activity.
Limitations of the current study include the cross-sectional design that prevents longitudinal assessment of nutritional status in cGVHD. Future studies that include newly diagnosed patients, provide longitudinal monitoring or incorporate monitoring of nutritional status during therapeutic interventions are needed. Challenges in conducting prospective clinical trials in cGVHD patients are well known.30,31 The current study presents an effective method for one-time evaluations when studying malnutrition in patients with a wide-range of cGVHD manifestations.
In summary, malnutrition is a significant complication for cGVHD patients and is associated with GI, lung and mouth manifestations and impaired functional status and QOL. Earlier diagnosis of nutritional deficits may be feasible using one-time evaluation by PG-SGA before weight loss detection by low BMI. Nutritional intervention studies should explore whether malnutrition could be prevented and lead to improved cGVHD outcomes.
Supplementary Material
Acknowledgments
This study was funded by the intramural programs of the National Cancer Institute, Center for Cancer Research and the National Institute of Dental and Craniofacial Research, as well as by the other intramural programs of the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ferrara J, Levine J, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syrjala K, Martin P, Lee S. Delivering care to long-term adult survivors of hematopoietic cell transplantation. J Clin Oncol. 2012;30:3746–3751. doi: 10.1200/JCO.2012.42.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsohn D, Margolis J, Doherty J, Anders V, Vogelsang G. Weight loss and malnutrition in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2002;29:231–236. doi: 10.1038/sj.bmt.1703352. [DOI] [PubMed] [Google Scholar]
- 5.Nixon DW, Heymsfield SB, Cohen AE, Kutner MH, Ansley J, Lawson DH. Protein-calorie undernutrition in hositalized cancer patients. Am J Med. 1980;68:683–690. doi: 10.1016/0002-9343(80)90254-5. [DOI] [PubMed] [Google Scholar]
- 6.Deeg HJ, Seidel K, Bruemmer B, Pepe MS, Appelbaum FR. Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transplant. 1995;15:461–468. [PubMed] [Google Scholar]
- 7.Kyle U, Chalandon Y, Miralbell R, Karsegard V, Hans D, Trombetti A, et al. Longitudinal follow-up of body composition in hematopoietic stem cell transplant patinets. Bone Marrow Transplant. 2005;35:1171–1177. doi: 10.1038/sj.bmt.1704996. [DOI] [PubMed] [Google Scholar]
- 8.Gupta D, Vashi P, Lammersfeld C, Braun D. Role of nutritional status in predicting the length of stay in cancer: a systematic review of the epidemiological literature. Ann Nutr Metab. 2011;59:96–106. doi: 10.1159/000332914. [DOI] [PubMed] [Google Scholar]
- 9.Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutritional tool in patients with cancer. Eur J Clin Nutr. 2002;56:779–785. doi: 10.1038/sj.ejcn.1601412. [DOI] [PubMed] [Google Scholar]
- 10.Leuenberger M, Kurmann S, Stanga Z. Nutritional screening tools in daily clinical practice: the focus on cancer. Support Care Cancer. 2010;S2:17–27. doi: 10.1007/s00520-009-0805-1. [DOI] [PubMed] [Google Scholar]
- 11.Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. Measuring therapeutic response in chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response criteria working group report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Cook E, Soiffer R, Antin J. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:444–452. doi: 10.1053/bbmt.2002.v8.pm12234170. [DOI] [PubMed] [Google Scholar]
- 13.Davidson M, Morton Nd. A Systematic review of the Human Activity Profile. Clin Rehabil. 2007;21:151–162. doi: 10.1177/0269215506069475. [DOI] [PubMed] [Google Scholar]
- 14.Ware J, Gandek B. Overview of the SF-36 health survey and the International Quality of Life Assessment (IQOLA) project. J Clin Epidemiol. 1998;51:903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 15.McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357–368. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell S, Leidy N, Mooney K, Dudley W, Beck S, LaStayo P, et al. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD) Bone Marrow Transplant. 2010;45:762–769. doi: 10.1038/bmt.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pidala J, Kurland B, Chai X, Majhail N, Weisdorf D, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the chronic GVHD consortium. Blood. 2011;117:5651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzberg P, Heussner P, Mumm F, Horak M, Hilgendorf I, Harsdorf Sv, et al. Validation of the human activity profile questionnaire in patients after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:707–1717. doi: 10.1016/j.bbmt.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Mehta C, Patel N. A network algorithm for performing Fisher’s exact test in r x c contingency tables. J Am Stat Assoc. 1983;78:427–434. [Google Scholar]
- 20.Agresti A. Categorical Data Analysis. John Wiley and Sons Inc; New York, NY, USA: 1990. [Google Scholar]
- 21.Grkovic L, Baird K, Steinberg S, Williams K, Pulanic D, Cowen E, et al. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012;26:633–643. doi: 10.1038/leu.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grkovic L, Mitchell SA, Baird K, Steinberg SM, Cowen EW, Williams KM, et al. Assessing the validity of the NIH response criteria for chronic graft-versus-host disease (cGVHD): consensus measures correlate with clinical outcomes. Blood. 2011;118 Abstract 4074. [Google Scholar]
- 23.Roberts S, Thompson J. Graft-vs-host disease: nutrition therapy in a clinically challenging condition. Nutr Clin Pract. 2005;20:440–450. doi: 10.1177/0115426505020004440. [DOI] [PubMed] [Google Scholar]
- 24.Lenssen P, Sherry ME, Cheney CL, Nims JW, Sullivan KM, Stern JM, et al. Prevalence of nutrition-related problems among long-term survivers of allogeneic marrow transplantation. J Am Diet Assoc. 1990;90:835–842. [PubMed] [Google Scholar]
- 25.Imanguli MM, Alevizos I, Brown R, Pavletic SZ, Atkinson JC. Oral graft-versus-host disease. Oral Dis. 2008;14:396–412. doi: 10.1111/j.1601-0825.2008.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imanguli MM, Atkinson JC, Mitchell SA, Avila DN, Bishop RJ, Cowen EW, et al. Salivary gland involvement in chronic graft-versus-host disease: Prevalence, clinical significance, and recommendations for evaluations. Biol Blood Marrow Transplant. 2010;xxx:1–8. doi: 10.1016/j.bbmt.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fall-Dickson JM, Mitchell SA, Marden S, Ramsay ES, Guadagnini JP, Wu T, et al. Oral symtom intensity, health-related quality of life, and correlative salivary cytokines in adult survivors of hematopoietic stem cell transplantation with oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:948–956. doi: 10.1016/j.bbmt.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenssen P. Hematopoietic cell transplantation: Contemporary Nutrition Support Practice. Saunders Co; St. Louis, MO, USA: 2003. [Google Scholar]
- 29.Stosovic M, Naumovic R, Stanojevic M, Simic-Ogrizovic S, Jovanovic D, Djukanovic L. Could the level of serum albumin be a method for assessing malnutrition in hemodialysis patients? Nutr Clin Pract. 2011;26:607–613. doi: 10.1177/0884533611419665. [DOI] [PubMed] [Google Scholar]
- 30.Martin PJ, Inamoto Y, Carpenter PA, Lee SJ, Flowers ME. Treatment of chronic graft-versus-host disease: past, present and future. Korean J Hematol. 2011;46:153–163. doi: 10.5045/kjh.2011.46.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12:491–505. doi: 10.1016/j.bbmt.2006.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


