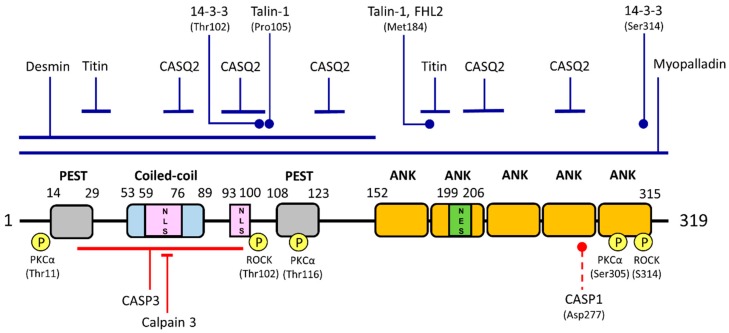
Figure 1.
Schematic representation of human ankyrin repeat domain 1 (ANKRD1) structure, binding partners and phosphorylation and cleavage sites. The ANKRD1 protein has 319 amino acids consisting of a coiled-coil domain, two PEST-like regions, an ankyrin repeat domain consisting of five ankyrin repeats (ANK), two putative nuclear localization signals (NLSs) and one potential nuclear export signal (NES). ANKRD1 has two binding sites for titin and five binding sites for calsequestrin (CASQ2) which are indicated by blue solid lines. Regions or residues involved in interaction with myopalladin, desmin, talin-1, four-and-a-half LIM domains 2 (FHL2) and 14-3-3 proteins are also indicated accordingly. Experimentally verified phosphorylation sites (yellow-circled P) and the protein kinases (PKCα, protein kinase C; ROCK, Rho kinase) responsible are indicated. Regions harboring cleavage sites by caspase-3 (CASP3) and calpain 3 are indicated by solid red lines. Predicted caspase-1 (CASP1) cleavage site is marked by a dashed line. Diagram is not drawn to scale.