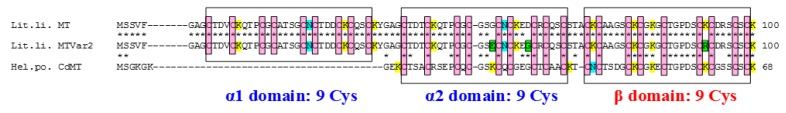
Figure 1.
Alignment of Littorina littorea Metallothionein (MT) (Lit.li. MT, GenBank Acc. No. AAK56498), with its allelic variant (Lit.li. Var2, GenBank Acc. No. KY963497), and with the paradigmatic Cd-specific MT isoform of the terrestrial snail, Helix pomatia (Hel.po. CdMT, GenBank Acc. No. AAK84863.1). Throughout, conserved cysteine (Cys) positions in the peptide chains are highlighted by a pink box. In the sequence of the allelic Lit.li. MTVar2, the amino acid positions exchanged with respect to the wildtype Lit.li. MT sequences are shown in green. Also highlighted in yellow are the Lysine (K) residues whose preponderance over Aspargine (N) (highlighted in blue) in the sequences is supposed to confer to the respective peptides a high Cd(II) binding preference [19]. The transparent boxes indicate the supposed three-domain structure of the three MT proteins, which has been experimentally verified by solving the structure of Littorina littorea MT [20]. According to this, the Helix pomatia CdMT consists of two modular domains (one α2 and one β domain), whereas the two MT variants of Littorina littorea comprise three modular domains (two α domains, i.e., α1 and α2, and one β domain). In each of the above-shown proteins, every domain includes 9 Cys residues that provide 9 sulfur atoms for the binding of 3 divalent metal ions (such as Cd(II)). Identical positions between adjacent sequences are indicated by stars. The number of residues in the respective peptide chains is specified near their C-terminal end.