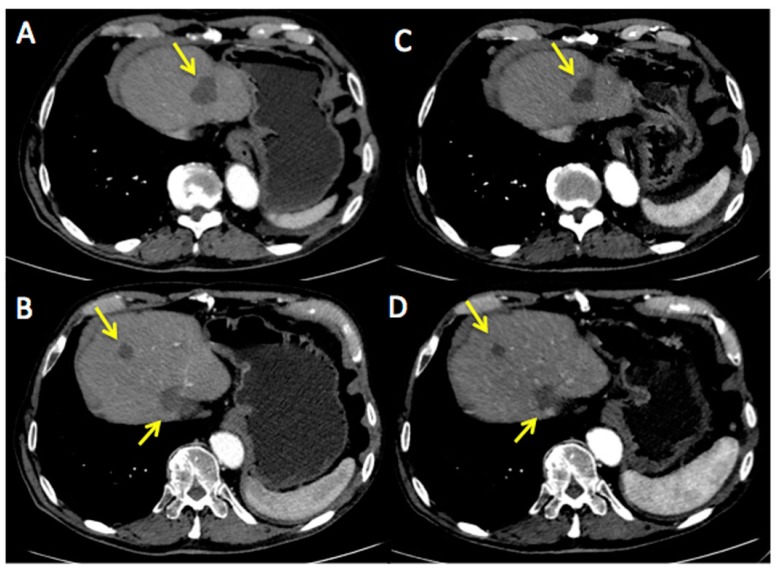
Figure 2.
A 71-year-old male affected by colorectal cancer with liver metastasis, subjected to 6 cycles of Bevacizumab-based chemotherapy and 10 hyperthermia sessions on the abdomen as second-line. Baseline Multidetector Contrast Enhancement Computed Tomography (MDCT) (A,B) showed metastasis in the II and VIII segment of the liver and caudate lobe (maximum diameter: 47 mm, yellow arrows). Timepoint-1 MDCT (C,D) evaluation demonstrated stable size of liver metastasis (maximum diameter: 44 mm, yellow arrows). According to mRECIST, patient was classified as SD. Ca19.9 and Carcino-Embryonic Antigen (CEA) values evaluated at baseline were 99 UI/mL and 65 ng/mL, respectively; while Ca19.9 and CEA values evaluated at timepoint-1 were 81 UI/mL and 60 ng/mL, respectively. Side effects reported were limited to nausea and mild position-related pain during HT sessions.