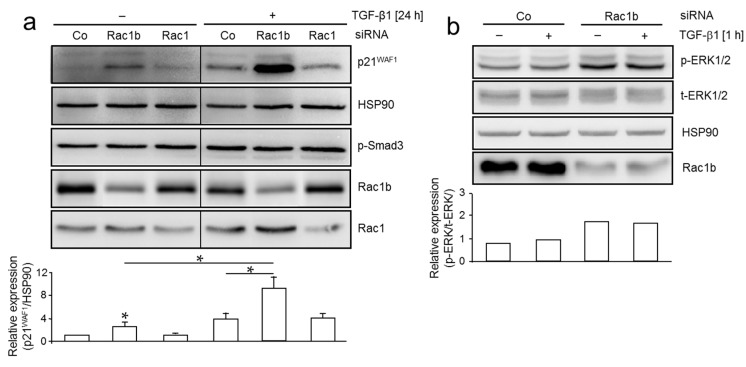
Figure 2.
Depletion of Rac1b in MDA-MB-231 cells increases TGF-β-induced p21WAF1 expression and ERK1/2 phosphorylation. (a) MDA-MB-231 cells were transiently transfected twice at two consecutive days with 50 nM of either a control siRNA (Co), a Rac1b-specific siRNA, or a Rac1-specific siRNA not cross-reactive with Rac1b. Twenty-four h after the second transfection, cells were starved (0.5% FBS) overnight and subsequently treated in the same medium with TGF-β1 for 24 h followed by immunoblot analysis of p21WAF1, phospho-Smad3 (p-Smad3), and HSP90 as loading control. Successful depletion of Rac1b and Rac1 protein was verified with a Rac1b-specific antibody and a Rac1 antibody, respectively. Onerepresentative blot is shown withall bands being from the same blot. The vertical lines between lanes of control and TGF-β-treated samples indicate removal of irrelevant lanes. The graph below the blot depicts results from a densitometric analysis using NIH Image J of underexposed replicates from three independent experiments (mean ± SD, n = 3). * p < 0.05; (b) MDA-MB-231 cells were transiently transfected twice at two consecutive days without transfection reagent alone (-), or with 50 nM of either a control siRNA (Co), or a Rac1b-specific siRNA and further processed as described in (a) except that treatment with TGF-β1 was for 1 h. Immunoblots were incubated with antibodies to phospho-ERK1/2 (p-ERK1/2), total-ERK1/2 (t-ERK1/2), and Rac1b and HSP90 as controls. The immunoblot shown is representative of three independent experiments.